Image

Mathew W. MacCumber, MD, PhD | The Rush Department of Ophthalmology is strongly committed to clinical research. The department actively engages in a variety of National Institutes of Health, National Eye Institute, FDA and industry sponsored clinical trials. Being leaders in clinical research is vital to enhancing the educational mission of the department at the resident and fellowship level. The department receives funding from the NIH, the Illinois Society to Prevent Blindness, the Juvenile Diabetes Research Foundation, Alcon Surgical, and Bausch & Lomb. Research activities are coordinated by Mathew W. MacCumber, MD, PhD, the associate chairman for research, Lulu Halig, OD, MS our Research Coordinator. Throughout the training period, support is available for resident and medical student research projects. |
-
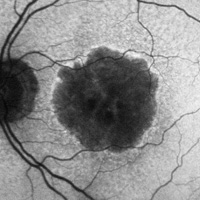
Age-related Macular Degeneration (AMD)Image
 Image
Image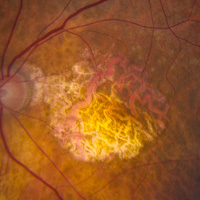
Dry AMD
- Apeliis, a phase 3, multicenter, randomized, double-masked, sham-controlled study to compare the efficacy and safety of intravitreal APL-2 therapy with sham injections in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). Primary Objective is to evaluate the efficacy of APL-2 compared to sham injection in patients with GA secondary to AMD assessed by change in the total area of GA lesions from baseline as measured by fundus autofluorescence (FAF).
- GEMINI-001, a Genetic Screening Study to Evaluate Long-term Clinical Outcomes in Subjects with Non-Central Geographic Atrophy (GA) Who Are Carriers of High-Risk Genetic Complement Variants Associated with Dry Age-related Macular Degeneration(AMD) GEM-NH-001-Cohort 0 is a prospective, multicenter, longitudinal registry, consisting of 375 participants with non-central geographic atrophy (GA) secondary to dry age related macular degeneration (AMD), who are carriers of known genetic variants associated with AMD. The primary objective of the GEM-NH-001 study is to develop a participant dry AMD registry which can allow for collection of substantial longitudinal data to further increase understanding of disease progression and to support future interventional studies.
ImageWet AMD
- Mako Study, phase 3 study of the Efficacy and safety of Squalamine Lactate Ophthalmic Solution 0.2% administered twice daily in subjects with neovascular age-related macular degeneration
-
FUCHS DystrophyImage
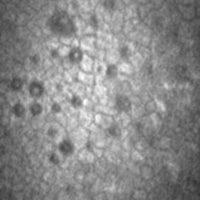
FUCHS, a double-masked, randomized, placebo-controlled, parallel-group, 12-week study to investigate the safety and efficacy of ripasudil (K-321) eye drops after descemetorhexisin patients with Fuchs endothelial corneal dystrophy
This is a multi-center, double-masked, randomized, parallel-group controlled 2-period study after descemetorhexis in patients with Fuchs endothelial corneal dystrophy.
-
Zoster Eye Disease Study (ZEDS)Image
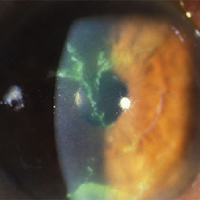
The objective of the Zoster Eye Disease study (ZEDS) is to determine whether prolonged suppressive oral antiviral treatment with oral Valacyclovir reduces the complications of Herpes Zoster Ophthalmicus (HZO) thereby improving the clinical outcome of this disease. The primary aim of this double masked placebo-controlled multicenter randomized clinical trial will test the hypothesis that suppressive antiviral treatment with oral Valacyclovir for 12 months will reduce the rate of new or worsening disaster corneal disease compared to placebo at the 12 month primary and point.
-
UveitisImage
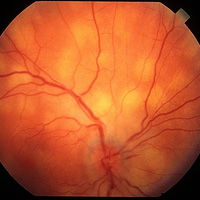
ADVISE, Adalimumab vs. conventional Immunosuppression for Uveitis Trial. Treatment of non-infectious, intermediate, posterior, panuvetitis. Adalimumab OR conventional immunosuppressive therapy. Randomized.
Image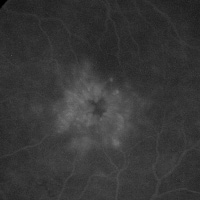 Image
Image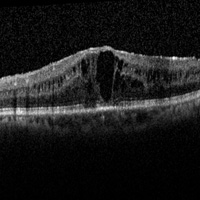
MERIT, Macular Edema Ranibizumab v. Intravitreal Anti-inflammatory Therapy Trial (MERIT). This study will compare the relative efficacy and safety of intravitreal methotrexate, intravitreal ranibizumab, and the intravitreal dexamethasone implant for the treatment of uveitic macular edema persisting or reoccurring after an intravitreal corticosteroid injection. MERIT is a parallel design (1:1:1), randomized comparative trial with an anniversary close-out at the 6 month clinic visit. The primary outcome is percent change in central subfield thickness from the baseline OCT measurement to the 12 week visit.
-
Fellow/Resident/Medical Student Ophthalmology Studies
Participant(s) Principal Investigator Title Sarah Carballo, MD ( PGY4), Grace Alexander
Pauline T. Merrill, MD Monitoring and Predicting Outcomes of Birdshot Chorioretinopathy: A Proposed Prognostic Matrix
Nabila Sardar, MD (PGY4) Pauline T. Merrill, MD Birdshot chorioretinopathy Emily Shepherd, MD (PGY3) Jack A. Cohen, MD Long term visual and neurodevelopmental outcomes in ROP patients treated with laser photocoagulation versus intravitreal anti-VEGF therapy Omar Dajani, MD (PGY6) John S. Pollack, MD Impact of Pre-Operative Optical Coherence Tomography (OCT) Abnormalities in Epiretinal Membrane (ERM) Patients on Post-Operative Anatomic and Visual Success: A Retrospective Study Naryan Sabherwal, MD Mathew W. MacCumber, MD, PhD Altitude retinopathy case
