The Tribology Laboratory, housed in the Department of Orthopedic Surgery, supports the medical industry in finding solutions related to lubrication mechanisms of joints, causes of articular cartilage degradation, friction and biocompatibility of orthopedic prostheses. Our aim is to improve the understanding of surface-surface interactions to reduce friction and wear of natural and artificial joints. This includes developing more durable bearing surfaces to extend their useful lifespan in patients and test new surface treatments for orthopedic implants.
Tribology is the science of interacting surfaces in relative motion, and it studies friction, lubrication and wear.
The Tribology Laboratory works closely with the Motion Analysis, the Computational Biomechanics, the Implant Pathology and the Spine Biomechanics laboratories.
Learn more about the lab:
The Tribology Laboratory was founded as a laboratory to provide biomaterials testing for medical research over 20 years ago. Over time our capabilities have expanded into a wide range of tests related to evaluating the joint implants. Our research can be grouped in the following areas:
Joint replacement procedures are currently carried out for hips, knees, shoulder, elbows, ankles and spinal disks. While most implants remain fully functional, with a survival rate of joint implants exceeding 90% after 17 years, nearly 10% of implants required revision surgery. Research on the lubrication and mechanical and chemical degradation are require to extend their implantation time.
-
Tribocorrosion of metals alloys
Artificial joint implants are used to relieve pain and restore function in degenerated joints caused by disease, trauma or genetic condition. The primary function of implants is tribological as the bearing surfaces articulate under load. However, all implantable metal alloys are also susceptible to corrosion to a certain degree, depending on the metallurgical condition, residual or service stresses and surface treatment applied prior to implantation. Corrosion can reinforce tribological damage and vice versa, this is called tribocorrosion. Therefore, artificial joints are susceptible to the usual tribology issues of high friction, wear, corrosion and fatigue and these problems can contribute to failure and revision. The actual corrosion resistance of a material can only be proven by long-term clinical trials, but accelerated laboratory tests can be used to predict certain effects. Understanding the complex physical and chemical interactions between contact surfaces and the surrounding body fluids is now an area of research in artificial joint tribology.
The role of interfacial reactions in the degradation of joint surfaces and junctions by tribocorrosion is studied in the laboratory. We have made substantial contributions to tribocorrosion research and to the understanding of synergies and antagonisms between wear and corrosion in the medical field. We remain active in all aspects of tribocorrosion research. Our activities include the following areas of interest:
- Evaluate the corrosion resistance of various medical alloys by simulating the in vitro inflammatory conditions.
- Address the clinical issue of inflammatory response to wear and corrosion products of metal orthopedic implants by combining in situ culture of cells in a biotribometer.
- Understand and characterize the tribochemical process of fretting corrosion on metals. Investigate the effects of microgrooves on the fretting-corrosion behavior of contemporary hip implants and determine the dominated degradation mechanism.
- Characterize how friction and implant wear are related to lubricant chemistry. Understand the influence of the synovial fluid components (proteins, bovine serum albumin, hyaluronic acid) on the tribological performance of metal alloy, as the electrochemical point of view.
- Analyze surface medical implant by focusing on tribocorrosion products, passive film and carbonaceous ‘tribofilm’.
-
Wear of polyethylene components
Polyethylene components are used as liners and bearing surfaces for joint replacements. The Ultra High Molecular Weight Polyethylene (UHMWPE) is the dominant bearing material of choice in Total joint Arthroplasty for its tribological and mechanical properties that can ensure long in vivo service. Cross-linked UHMWPE greatly reduced wear, and particle biocompatibility without causing catastrophic ruptures and tissue reactions.
New and promising materials, like crosslinked and vitamin E charged polyethylenes, are considered safe but innovative and are therefore handled cautiously. Many in vitro tests and several in vivo demonstrations have confirmed the validity of these materials, but it is important to remember that they do not yet have long-term clinical histories. In the Tribology Laboratory, we are investigated the role of debris of crosslinked polyethylene, the quantity and reactivity of which are still to be elucidated and the long-term behavior of cross-linked material. The accurate documentation of the relative wear performance of well-characterized contemporary and historic polyethylene is important both in the projection of the future success of polyethylene-based implant treatment and in the consideration of alternative bearing types. Our interest covers the following topics:
- Evaluate the wear of UHMWPE with joint simulators.
- Develop new methods to measure polymer wear of artificial joints based on the wear and damage score (delamination, cracking, significant deformation).
- Design a custom made joint simulator to investigate the response of knee joints under representative loads.
- Characterize the wear particles in metal-on-polyethylene joints and evaluate the specific immune response, early adverse tissue responses, and genotoxicity.
- Develop a model to consider the individual gait bears on wear rate from gait biomechanics [Collaboration with the Motion Gait Lab].
- Analyze post clinical retrieval analysis of polyethylene tibial components of total knee implants.
-
Hemiarthroplasty
Hemiarthroplasty is a procedure to replace degenerative and non healthy articular cartilage by implant material that will articulate against it. It is a surgery less invasive than total joint arthroplasty and preserve the cartilage and bone capital of patients.
The friction and lubrification of hemi implants articulated against articular cartilage is important to be investigated in laboratory in order to extend their lifetime and therefore postpone the implantation of total implant. We have the ability to conduct non stationary mechanical stimulation of live cartilage and complement it with biological analyses to understand the in vitro reaction of cartilage. Our research interests are to:
- Analyze the mechanical and frictional response of different shoulder humeral component materials against the natural glenoid.
- Evaluate the wear and degradation of live cartilage in a tribological interface.
- Quantify biomarkers of the cartilage degradation such as proteoglycans and hydroxyproline.
- Evaluate the viability and metabolic activity of chondrocytes after mechanical stimulation of the cartilage tissue.
The preservation of articular cartilage is highly dependent on maintaining its organized architecture. A fundamental understanding of articular cartilage mechanics is essential for aiding clinicians in enhancing the longevity of healthy joints and rehabilitating injured joints.to the development of promising treatment.
-
Topic
Articular or hyaline cartilage is a thin layer of specialized connective tissue that covers the ends of bones. Its primary function is to provide low friction and wear under normal function and to facilitate the transmission of loads to the underlying subchondral bone. When hyaline cartilage begins to degenerate with age, or when it is damaged by injury, the tissue may be overstressed. The injury and resulting loss of function can increase friction, signal further matrix degeneration and initiate osteoarthritis (OA).
Unlike most tissues, articular cartilage is devoid of blood vessels, nerves, or lymphatics. It is composed of a dense extracellular matrix (ECM) with a sparse distribution of highly specialized cells called chondrocytes. The ECM is principally composed of water, collagen, and proteoglycans, with other non-collagenous proteins and glycoproteins present in lesser amounts. Together, these components help to retain/attract water, which is critical to maintain its unique mechanical properties.
From a mechanical point of view, articular cartilage is a composite of materials composed of a fluid phase (mainly water) and a solid phase (ECM). Cartilage is considered a permeable, viscous and elastic material. Due to its elasticity, articular cartilage gives rise to deformations, but always returns to its original shape, and it combines a remarkably long life with low friction. Under dynamic loads, a sort of pumping effect occurs. This leads to an increase in volume and also causes an increase in tension in the collagen fibers, which increases the elasticity of the tissue. Collagen fibers provide good tensile strength. The water-proteoglycan compounds provide compressive strength and elasticity.
The biological and mechanical properties of articular cartilage are very complex and vary from area to area depending on its composition (water concentration, proteoglycan content, collagen fibers orientation). The preservation of articular cartilage is highly dependent on maintaining its organized architecture. A fundamental understanding of articular cartilage mechanics is essential for aiding clinicians in enhancing the longevity of healthy joints and the development of promising treatments for injured joints.
-
Current research area
Our group is investigating the unique properties of articular cartilage such as the intrinsic viscoelasticity, the wear resistance and the stiffness. The current interesting research area are focusing on:
- Study the effects of physiological sliding activity (repeated loading and shearing) on the biomechanical and biological homeostasis of articular cartilage.
- Explore the effect of articular cartilage degeneration and property changes on tribological and mechanical function.
- Investigate and assess the potential of early stage treatments for osteoarthritis.
- Perform long-term controlled studies of live cartilage biomechanics and mechanobiology under physiologically consistent sliding conditions.
- Correlate mechanically/enzymatically damaged and healthy cartilage
- Quantify markers characteristic of the biochemical response of cartilage tissue, and observe any structural changes by histological analysis.
- Evaluate the collagen fibers resistance (tensile strength) by tensile test.
- Assess the intrinsic viscoelastic properties and the stress-relaxation response of articular cartilage by nanoindentation.
Infection on and around an artificial implant prosthesis, such as knee or hip replacement surgery can be a devastating and costly complication. It is amongst the leading causes of revised surgery and poor surgical outcomes in orthopedics.
-
Topic
Biomaterial-associated infections are a result of microorganisms adhering to a medical implant or device. Following adherence of the microorganism to the implant or medical device, biofilm formation can occur. Development of biofilm on an implant surface makes these infections highly resistant to immune host defense and antimicrobials. This resistance makes these biomaterial-associated infections difficult to treat. These types of infections can be challenging to manage and result in:
- Requirement of additional surgeries and implant removal and replacement;
- Requirement of prolonged antibiotic treatment;
- Additional medical complications for the patient; and
- High medical costs and burden for the healthcare system.
Our team focuses on methods of prevention and treatment of periprosthetic joint infection (PJI), which is infection on and around an artificial implant prosthesis, such as those used in knee or hip replacement surgery. PJI can be a devastating and costly complication of joint replacement surgery and is amongst the leading causes of revised surgery and poor surgical outcomes in orthopedics.
-
Current research area
Building on the experience of conducting sterile lab tests and corrosion research, our group investigates the following areas:
- Local delivery of antimicrobials to the implant site, such as through coating the implant with antimicrobial agents. This is in contrast to common systemic oral or intravenous antibiotic utilization, which can result in: i) low bioavailability of antimicrobial drug around the implant and surgical site; and ii) toxic side effects on normal body tissues and organs at high doses.
- Creating microscopic size pockets (nanotubes) on the surface of metal implants to enhance drug loading at the implant site and further optimizing the drug release from these nanotubes.
- Promotion of sustained local bioavailability of antimicrobials on and around the implant through utilization of controlled release materials.
- Utilization of agents targeting prevention of development and disruption of biofilm.
- Preclinical animal models of PJI.
New publications
- Impergre A, Trunfio-Sfarghiu A-M, Wimmer MA, Evaluation of articular cartilage wear against pyrolytic carbon in the context of spherical interposition shoulder arthroplasty, Biotribology Journal, Volumes 33–34, 2023, 100237 Link
- Hamilton JL, Vashi M, Kishen EB, Fogg LF, Wimmer MA, Balk RA. The Association of an Alpha-2 Adrenergic Receptor Agonist and Mortality in Patients with COVID-19. Front. Med. 2022 Jan; 8:797647 Link
- Queiroz Neto M, Radice S, Hall DJ, Frisch NB, Mathew MT, Fischer A, Jacobs JJ, Pourzal J; Microstructure and electrochemical behavior of contemporary Ti6Al4V implant alloys. Journal of Bio- and Tribo-Corrosion 8, 26 (2022) Link
Past presentations
- Koya T, Sato A, Oike J, Ota M, Izukash K, Okumo T, Yagura S, Yokohama, Okuma N, Kawashima F, Takagi H, Kanzaki K, Accuracy of the Component Alignment and Sizing in Total Knee Arthroplasty: Preoperative Templating and Postoperative Evaluation Using CT-Based 3D Pre-Operative Planning Software, 14th Biennial ISAKOS Congress, Boston, MA, June 18-21, 2023
- Impergre A, Wimmer MA, Are sGAGs transferred onto the surface of hemiarthroplasty implants while rubbing against cartilage? 2023 Society For Biomaterials, San Diego April 19-22, 2023
- Impergre A, Wimmer MA, Affinity of Phospholipids Vesicles with Pyrolytic Carbon Shoulder Implant” at the 243rd ECS Meeting - 18th International Symposium on Solid Oxide Fuel Cells (SOFC-XVIII), May 28-June 2, 2023, Boston, MA
Orthopaedic Research Society Annual Meeting, Dallas, TX, February 2023
- AbuAlia MJ, Wimmer MA, Articular cartilage shear properties change with compression and are dependent on an intact superficial zone.
- Markovics A, Hamilton JL, Della Fara G, AbuAlia MJ, Impergre A, Wimmer MA. Electrophoretic Deposition of Gentamicin Into Titanium Nanotubes to Prevent and Eradicate Periprosthetic Infection.
7th World Tribology Congress 2022, Lyon, France
- Impergre A, Sfarghiu AM, Wimmer MA, Comparative study of pyrolytic carbon, zirconia-toughened alumina, and cobalt-alloy against cartilage: in vitro wear test with live tissue.
- Fischer A (stepping in for Wimmer MA) Wear and Repassivation Rates of Orthopedic Implants in Simulated Healthy and Inflammatory Synovial Fluids.
- Fischer A, Wear scars do not represent wear loss – a fretting corrosion example.
- Impergre A (stepping in for Wimmer MA) Fullam S, Schmid T, Yuh C, Witt B, Wimmer MA, Zinc Distribution in Articular Cartilage and Potential Implications on Structural Role.
33rd Annual Congress of International Society for Technology in Arthroplasty, Maui, HI, USA
- Hamilton J, Markovics A, Della Fara G, Impergre A, Wimmer MA, Use of Electrophoretic Deposition in the Hospital Setting to Coat Medical Implants with Therapeutic agents to Prevent or Treat Biomaterial Associated Infection.
- Impergre A, Wimmer MA, Ben Trad L, Bernoud-Hubac N, Sfarghiu AM, Tribological Evaluation of a Pyrolytic Carbon Interposition Implant Against Live Cartilage in Comparison with Alumina Ceramic and CoCrMo-Alloy.
- Della Fara G, Markovics A, Hamilton JL, Wimmer MA. Electrophoretic Deposition of Gentamicin and Chitosan Into Titanium Nanotubes to Prevent Periprosthetic Joint Infection.
Awards
- Alfons Fischer received the Georg Vogelpohl Award from the German Society of Tribology (https://www.gft-ev.de/en/honours/)
Our team consist of experts in materials, mechanics, chemistry, and biology.
-
Faculty, Postdoctoral Fellows and StaffImage

Markus A. Wimmer, PhD
DirectorProfessor Wimmer is the Grainger Director of the Rush Arthritis and Orthopedics Institute in Chicago and serves as the Associate Chairman for Research in the Department of Orthopedic Surgery at Rush University Medical Center. He holds conjoint appointments in the Departments of Rheumatology, Anatomy and Cell Biology, as well as Biomedical Engineering at the University of Illinois at Chicago.
Dr. Wimmer received his diploma in Mechanical Engineering from the Technical University of Munich, Germany. After a post-graduate year in Chicago, Dr. Wimmer continued his education in Germany and earned a doctorate in Biomechanics at the Hamburg University of Technology. Before joining Rush in 2001, he spent four years at the AO Research Institute in Davos, Switzerland.
Image
Thomas M. Schmid, PhD
Associate Professor EmeritusDr. Schmid is a Biochemist and Cell Biologist. His research career has focused primarily on biomarkers in cartilage. He discovered type X collagen and showed it is a biomarker for hypertrophic chondrocytes at the interface of calcified and uncalcified cartilage. The synthesis of type X collagen is induced by Ca(+2) ions. He also identified superficial zone protein (lubricin) as a biomarker at the surface of articular cartilage where it is synthesized by superficial zone chondrocytes. It acts as a major lubricant in synovial joints by lowering the coefficient of friction at this surface. Its synthesis is induced by TGFa released as one tissue rubs against another.
Image
Amandine Impergre, PhD
Laboratory SupervisorAmandine Impergre is the Tribology Laboratory Supervisor in the Department of Orthopedic Surgery at Rush University Medical Center.
Her research areas include corrosion, tribocorrosion and biocompatibility of metal biomaterials, with a focus on preventing accelerated chemical degradation and cytotoxicity of cartilage. She completed her Bachelor of Science degree in Physics and Chemistry and her Master in Corrosion and Materials engineering at the University of La Rochelle, in France. She graduated first in her class with the highest GPA. She pursued her Ph.D. at MATEIS Laboratory in INSA of Lyon, France under the mentorship of Dr. Normand, a preeminent electrochemist scientist who is world-renowned for the corrosion characterization of metals and particularly in tribocorrosion.
Dr. Impergre completed her postdoctoral studies at Rush investigating the in vitro biotribological properties of the Pyrolytic Carbon implant (InSpyre), a non-metallic shoulder interposition implant, which minimize the replacement of healthy tissue in the joint. Since joining Rush in 2019, Dr. Impergre has brought knowledge of electrochemistry in the biomedical field, and lead biocompatibility assays of cartilage.
Image
John L. Hamilton, MD, PhD
InstructorJohn L. Hamilton is an Instructor in the Department of Orthopedic Surgery at Rush. He received his MD, PhD, and postdoctoral training at Rush University Medical Center. During this training, he was awarded an NIH F31 Predoctoral Fellowship in the Department of Biochemistry at Rush and investigated underlying mechanisms of osteoarthritis pathology as well as development of novel osteoarthritis treatments.
As an NIH T32 Postdoctoral Fellow in the Departments of Orthopedics, Internal Medicine, and Anatomy and Cell Biology at Rush, he investigated methods of immunomodulation to prevent periprosthetic joint infection. Current research efforts include translational projects in the fields of periprosthetic joint infection, degenerative joint disease, inflammation and immunology, and COVID-19.
Image
Mohammed J. AbuAlia, MD
Postdoctoral Research FellowMohammed is a postdoctoral research fellow in the Department of Orthopedic Surgery at Rush. He is a veteran who received his MD degree from St. George’s University School of Medicine in 2022, and his Chemistry Bachelor’s Degree from the University of Illinois at Chicago in 2014.
While still in medical school, he was awarded an NIH T32 Fellowship research training grant in joint health in the Department of Orthopedic Surgery at Rush. His past employemnt as an automotive mechanic provided him with extensive experience in mechanics. He also worked in the biochemisty department at the University of Illinois at Chicago. Current research work includes wear simulation and modeling, and investigating the rheological properties and behavior of bovine knee cartilage to help in understanding the mechanisms and progression of osteoarthritis, which is key for prevention and for optimal design of replacements.
Image
Adrienn Markovics, MD, PhD
Assistant ProfessorAdrienn Markovics MD, PhD is an Assistant Professor at the Department of Orthopedics. Her area of interest includes immunology, orthopedics and pharmacology. She holds a medical degree from the University of Pecs, Hungary, where she was awarded a PhD scholarship in pharmacological sciences. Dr. Markovics joined Rush University Medical Center in 2014 and investigates joint-related pathophysiology such as periprosthetic joint infection and the autoimmune rheumatoid arthritis. She is a member of The American Association of Immunologists and the Orthopaedic Research Society. She has been published in several peer-reviewed journals, her complete list of bibliography can be found at this link.
Image
Alfons Fischer, PhD
Assistant Professor, Visiting ResearcherAlfons Fischer received his Dipl.-Ing. degree in mechanical engineering from the Ruhr Universitat Bochum, Germany in spring of 1980. He completed his Dr.-Ing. (Ph.D.) and Priv.-Doz. (Private Lecturer) degrees in materials science and engineering from the same university in summer of 1984 and in fall of 1992, respectively.
From 1992 to 1996, he was head of quality management as well as vice-head of the center of analyses and testing of NuTech GmbH, Neumünster, Germany; a company providing laser-processing technologies, materials testing, and failure analyses.
Since 1996, he was a full professor for materials science and engineering at the University of Duisburg-Essen in Germany from which he retired February 2019. His research (fundamental and applied) as well as his services (materials development and testing, failure analyses, expert reports) focus on fatigue, wear and corrosion of metals and metal-matrix composites with institutional and industrial partners in mechanical, production, automotive, off-shore, tooling, and biomedical engineering.
Since 2005, he is a visiting researcher at Rush University Medical Center, Dpt. of Orthopedic Surgery, Chicago IL, USA and since March 2019 also at the Max-Planck-Institut für Eisenforschung, Duesseldorf, Germany. He serves as co-editor-in-chief of the archival journal WEAR since Jan. 1st 2020. He has published more than 240 papers and co-authored five books.
ImageTakayuki Koya, MD, PhD, Postdoctoral trainee
Visiting ResearcherDr. Koya is an orthopedic surgeon who has specialized in knee surgery in Japan. While working at the Showa University Koto Toyosu Hospital and Fujigaoka Hospital, he treated knee injuries and osteoarthritis by performing joint repairs such as ligament reconstructions and meniscus repairs and joint preserving surgeries such as osteotomies and arthroplasty procedures (TKA, UKA). Since April 2022, he has joined the Tribology and Motion Analysis Laboratories at Rush University as a Postdoctoral Trainee under Dr. Wimmer. His current research focuses on the influence of biomaterials articulating against cartilage to improve hemiarthroplasty and studying TKA function during recreational activities such as golf.
Image
Francesca De Vecchi, BS
Research AssistantFrancesca de Vecchi is a Biomedical Engineering graduate student enrolled in a double degree program between University of Illinois at Chicago and Politecnico of Milan. She achieved her bachelor’s degree in Biomedical Engineering at Politecnico of Milan and continued her studies there with a master’s degree focused on Biomechanics and Biomaterials.
In August of 2021, she moved to University of Illinois Chicago to complete the double degree program in the US. There, she joined the Tribology Laboratory at Rush University to work on her thesis, which she defended in December of 2022. Her research focuses on the tribological and mechanical characterization of bovine articular cartilage after chemical treatments to study the influence of divalence ions on the tissue.
-
Students of the past years
- Sofia Gianotti, Politecnico di Milano, Italy
- Francesca De Vecchi, Politecnico di Milano, Italy
- Paul Vanhalst, École des Mines de Saint-Étienne, France
- Saskia Heermant, Technische Universitat Dortmund, Germany
- Cristian Beckmann, Technische Universitat Dortmund, Germany
- Greta Della Fara, Politecnico di Milano, Italy
-
Alumni
2016 - 2021
Name Period Academic School Research Project Leora Cramer
2020-2021
Brandeis University
Concurrent validity and test retest reliability of instrumented shoe insoles
Jade He
2016-2021
Rush Univ., Biomechanics
Gait Retraining of Individuals with Medial Knee OA
Catherine Yuh
2015-2020
Rush Univ., Biotechnology
Investigation of articulation-induced articular cartilage stiffening
Filippo Cinotti
2019-2020
Politecnico di Milano, Italy
Assessment of TiN coatings for TJR
Lorenzo Girotto
2019-2020
Politecnico di Milano, Italy
Surface modification of titanium to combat PJI
Jacqueline Simon
2013-2019
Rose-Hulman Institute of Technology
Functional assessment of bicruciate total knee replacement
Chris Knowlton
2009-2019
Univ. of Illinois at Urbana-Champaign
Volumetric wear determination of retrieved knee implants
Taylor Holcomb
2016-2018
Cornell University
Full-scale design of a novel tribo-corrosion cell culture system
Steven Mell
2013-2018
University of Illinois at Chicago
A computational framework for parametric evaluation of TKR wear
Robert Trevino
2011-2016
Pace University
Role of mechanical wear in healthy cartilage
2010 - 2015
Name Period Academic School Research Project Yigiterkut Canpolat
2014-2015
University of Duisburg-Essen
Knee wear tests according to two different ISO standards
Ryan Freed
2013-2015
Clemson University
Translation motion analysis data to TKR simulator for walking and stair activities
Christoph Daniels
2013-2014
University of Duisburg-Essen
Patient specific TKR wear simulation and cross-validation with retrieved polyethylene liners
Elmira
2013-2015
Shahid Bahonar Univ of Kerman
Volumetric wear assessment and characterization of tibial inserts
Johnny Dufils
2013-2014
Ecole Centrale Lyon
Design and setting up of a corrosion fretting apparatus to investigate the effect of molybdate ions
Robert Cichon
2011-2012
University of Duisburg-Essen
Design of a new bioreactor test rig for tribo-corrosion studies
Bojan Mitevski
2011-2012
University of Duisburg-Essen
Wear analysis and patient data correlation of cruciate retaining total knee replacements
Diego Orozco
2006-2012
University of Colima, Mexico
Activities of Daily Living and Their Impact on Total Knee Replacement Wear
Jonathan Stoia
2010-2011
University of Illinois at Chicago
Cartilage articulating system as a wear benchmark for artificial joint replacement
Bryan Schlink
2009-2011
Purdue University
Gait biomechanics and disease severity in hip osteoarthritis
Patrick Werner
2010-2011
Technical University of Munich
Development and evaluation of gait retraining for patients with knee osteoarthritis using telemetric shoe insoles with tactile and audio feedback
Felix Liedtke
2010-2011
University of Essen-Duisburg
Generation of CoCr mechanically mixed zones and the tribological behavior under reverse sliding
Christian Schoss
2010-2011
University of Duisburg-Essen
Wear tests of a surface structured CoCrMo-alloy
2000 - 2010
Name Period Academic School Research Project Chris Ferrigno
2011-
Medical College of Georgia
The effect of bio feedback on knee adduction moment
Yuen-Ying Valentina Ngai
2006-2010
University of Manitoba;
Assessment of in vivo gait patterns on wear of total knee replacements
Yasha Dwivedi
2007-2009
Vishwakarma Institute of Technology
The influence of proteins on the wear of UHMWPE
Idubijes Rojas*
2007-2009
University of Illinois at Chicago
Dynamic surface EMG of TKR patients:
Luis Antonio Gallardo
2007-2009
Instituto Tecnologico y de Estudios Superiores de Monterrey
Oxidative stability of ultra-high molecular weight polyethylene doped with europium stearates
Vivek Shekhawat
2004-2009
Nagpur University, India;
Influence of Kinematics on Mechano-Biological Response of Articular Cartilage – An in vitro Investigation
Thorsten Schwenke
2003-2008
Hamburg University of Technology
Wear of the Tibial Polyethylene Component in Total Knee Replacements - The Significance of Sliding Velocity During Gait
Richard Kang*
2006-2007
Northwestern University
A Controlled Laboratory Study of the Biologic Effects of Repeated and Varying Loads on Osteochondral Grafts
Andrea Swanson*
2005-2007
Northwestern University
In vivo methods for locating the tibio-femoral contact pathway in TKR during gait
Christiane Schulz
2006-2007
Univ. Duisburg-Essen
Fretting of UHMWPE against stainless steel
Robin Pourzal
2005-2006
Univ. of Duisburg-Essen
Determination of polyethylene wear location and volume in well functioning acetabular cups
Diego Orozco
2004-2006
University of Colima, Mexico
An artificial neural network approach to understand wear of TKR
Laura Thorp*
2002-2006
University of Scranton, PA
The relationship between structure and function in knee osteoarthritis: a mechanical perspective utilizing gait analysis
Sidhart Nileshwar
2003-2005
University of Mumbai
Relating surface damage of articular cartilage to local mech properties
Maximilian Müller
2004-2005
Tech Univ. Munich
The role of biomechanical forces on cell deformation
Sascha Müller
2004-2005
Tech Univ Chemnitz
Cartilage stiffness and related stress during impaction grafting of osteochondral tissue
Rachna Sah
2004–2005
MGM College, Mombai, India
The role of biomechanical forces on cell deformation (Bioengineering)
Laura Borgstede
2002-2004
Colorado State University
Parametric analysis of wear mechanisms in total knee replacement
Priyanka Paul*
2002-2004
Univ. of Illinois at Urbana-Champaign
Wear characterization of retrieved total knee implants
Bill Nechtow*
2002-2004
Univ. of Illinois at Urbana-Champaign
Activity of total knee replacement patients
Tamara Pylawka*
2003-2004
University of Illinois
Role of Nitric Oxide in Metabolic Function of Cold Preserved Allograft Cartilage
Mozammil Hussain
2001-2003
University of Chandigarh
Stresses in polyethylene tibial inserts for two walking patterns using FEM
Staff
Name Period Role Spencer Fullam 2016-2021 Laboratory Supervisor Carol Pacione 2013-2016 Laboratory Supervisor Tobias Uth 2009-2013 Laboratory Supervisor
The tribology laboratory is equipped with a full range of friction/wear tests and joint simulators that simulate the forces and fluid environment of joints. Researchers also have the ability to perform electrochemical and tribocorrosion tests to study the corrosion resistance of orthopedic implants. Various surface characterizations and analyses are available to complete the evaluation of the in vivo behavior of orthopedic implants and the failure of joints in the human body.
The tribology laboratory also has the capability to perform friction tests in contact with cells/tissues such as cartilage explants. The biocompatibility or biological response of tissues undergoing mechanical testing (micro-, nano- and macro-) is assessed and complemented by a variety of biochemistry techniques.
-
Corrosion/Tribocorrosion/Biotribocorrosion devices
Several tribometers are available to evaluate the resistance to wear and corrosion of orthopedic implants. Direct contact with cell cultures is possible thanks to a tribometer housed in an incubator.
- Custom fretting corrosion simulator
- Six station pin-on-disc apparatus (Orthopod, AMTI Inc.)
- Tribocorrosion bioreactor in an incubator
- Several specifically dedicated hydraulic, pneumatic and electro-mechanic wear testing machines
- Potentiostats for corrosion and electrochemical testing (Gamry Instruments)
Image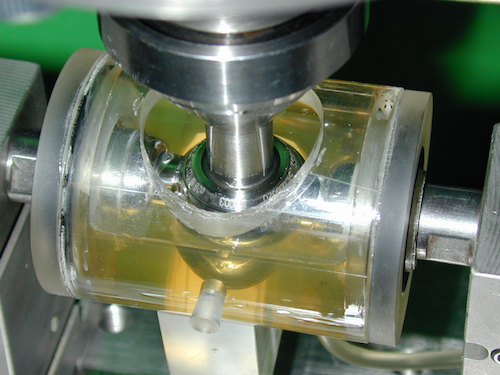
Detail of the fretting corrosion chamber.
Image
Tribocorrosion bioreactor.
Image
Six stations pin-on-discs apparatus.
-
Joint simulators
- Two four station knee/ankle motion simulators (EndoLab GmbH)
- A multifunction spine and hip simulator (EndoLab GmbH)
- Four-station hip joint simulator
Image
Knee joint simulator with four stations. Image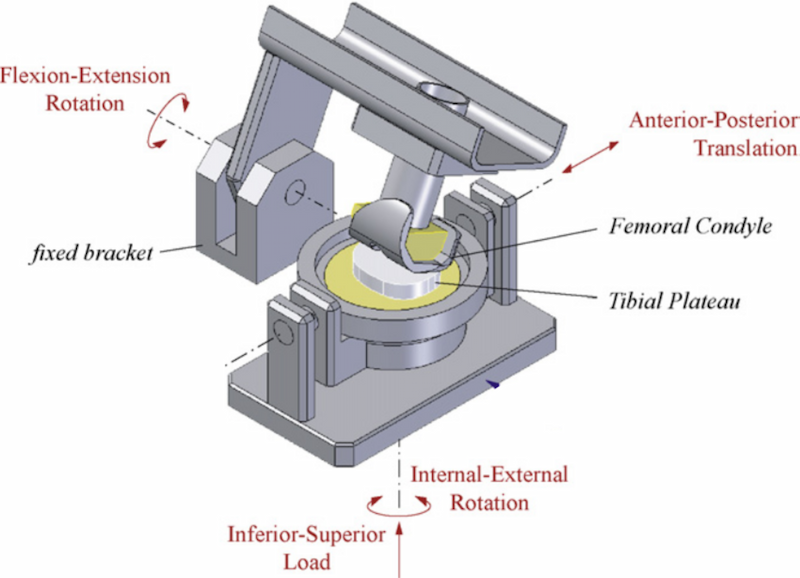
Mechanical view of the knee joint simulator. Image
Example of a knee implant. -
Cartilage testing
- Custom cartilage shear simulator
- Custom cartilage damage impactor
- Custom tensile test for cartilage
- Modular compact Rheometer, MCR302e (Anton Paar)
Image
Custom cartilage damage impactor Image
Custom cartilage shear impactor housed in an incubator Image
Modular compact Rheometer -
Biological equipments
Our group has the infrastructure on site, such as a Biohazard Safety Level 2 room. This room is located on the 7th floor of the Cohn Research Building. The Cohn Research Building is suitable to perform both microbiological and rodent experiments. Our equipment includes but is not limited to the following:
- In Vivo Imaging System (IVIS® Lumina II, Perkin Elmer)
- Spectrophotometer (Nanodrop 2000 UV-Vis spectrophotometer, Thermo Scientific)
- Biosafety cabinet, incubators and centrifuges
-
Characterization facilities - Surface analysis
- Ti-950 Nanoindenter (Hysitron, Inc.)
- Fourier Transform Infrared (FTIR) spectrometer: Cary 670 (Agilent Technologies)
- Confocal Raman Microscope, LabRAM HR Evolution (Horiba)
- 3D Surface Profiler VK-X3000 series (Keyence)
- Flash 250 SmartScope laser coordinate measuring machine (OGP, Inc.)
- Scanning electron microscope (SEM, JEOL IT500HR, Peabody) equipped with an Energy Dispersive Spectrometer detector (EDS, 65 Ultim Max detector, Aztec 4.1 SP1 software, Oxford Instruments)
Basic laboratory equipment are also available such as ultrasound cleaner, balances, magnifying glasses, pH-meter, centrifuge, water purification, chemical fumehoods, biosafety cabinet, sputter coater for sample preparation, etc.
Image
Ti-950 Nanoindenter
Image
Fourier Transform Infrared (FTIR) spectrometer
Image
Confocal Raman Microscope
Image
3D Surface Profiler
Image
Scanning electron microscope (SEM)
-
2022 Publications
- Radice, S., Queiroz Neto, M., Fischer, A., Wimmer, M.A. (2022) Nickel-Free High-Nitrogen Austenitic Steel Outperforms CoCrMo-Alloy regarding Tribocorrosion in Simulated Inflammatory Synovial Fluids. J Orthop Res 40(6):1397-1408
- Radice, S., Tibbits, G., Lin, A.Y.W., Beyenal, H., Wimmer, M.A. (2022) Interactions between hyaluronic acid and CoCrMo alloy surface in simulated synovial fluids. Biosurface and Biotribology 7(4): 239-250. DOI: 10.1049/bsb2.12027
- Hamilton, J.L., Vashi, M., Kishen, E.B., Fogg, L.F., Wimmer, M.A., Balk, R.A. (2022) The Association of an Alpha-2 Adrenergic Receptor Agonist and Mortality in Patients with COVID-19. Frontiers in Medicine 8: 797647. 10.3389/fmed.2021.797647
- Mell, S.P., Wimmer, M.A., Jacobs, J.J., Lundberg, H.J. (2022) Optimal surgical component alignment minimizes TKR wear – An in silico study with nine alignment parameters. J Mech Behav Biomed Mater 125: 104939. 10.1016/j.jmbbm.2021.104939
- Thampi, P., Tabbaa, S.M., Silbermans, G., McIlwraith, C.W., Laurent, M.P., Wimmer, M.A., Johnstone, B., Frisbie, D.D. (2022) Articular cartilage surface changes using surface topography in a post-traumatic equine model of osteoarthritis J Orthop Res 40(6):1349-57
- Cramer, L.A., Wimmer, M.A., Malloy, P., O’Keefe, J.A., Knowlton, C.B., Ferrigno, C. (2022) Validity and Reliability of the Insole3 instrumented shoe insole for ground reaction force measurements during walking and running. Sensors 22(6): 2203.
- Chastain, K., Wach, A., Pekmezian, A., Wimmer, M.A., Warren, R., Torzilli, P.A., Chen, T., Maher, S.A. (2022) ACL transection results in a posterior shift and increased velocity of contact on the medial tibial plateau. J Biomech.144:111335
- Odehnal, L., Ranuša, M., Wimmer, M.A. Vrbka, M., Křupka, I. (2023) Development of lubrication film and influence on friction in a total knee replacement during a gait cycle. Tribol. Int. 178:108073
- Ganguly, A., Olmanson, B.A., Knowlton, C.B., Wimmer, M.A., Ferrigno, C. (2023) Accuracy of the fully integrated Insole3’s estimates of spatiotemporal parameters during walking. Med Eng & Physics 111:103925
- Impergre, A., Trunfio-Sfaghiu, A.M., Wimmer, M.A. (2023) Evaluation of articular cartilage wear against pyrolytic carbon in the context of spherical interposition shoulder arthroplasty. Biotribology 33-34:100237
-
2022 Presentations
- S. Maher, A. Pekmezian; A. Wach, T. Chen, K. Chastain, J. Ruzbarsky, M.A. Wimmer, R.F. Warren, P.A. Torzilli. ACL Transection Results In A Posterior Shift And Increased Velocity Of Contact Most Prevalent In The Medial Compartment. Transactions Vol. 47: 67; Annual Meeting of the Orthopaedic Research Society, Tampa, FL, 2022
- C. Yuh, S. Chubinskaya, S. Maher, M.A. Wimmer. Characterization Of Surface Stiffness And Articular Loading-Induced Surface Stiffening In Human Ankle Cartilage. Transactions Vol. 47: 616; Annual Meeting of the Orthopaedic Research Society, Tampa, FL, 2022
- D. Gokul, E. Birch, K. Cheng, M. Mathew, D. Bijukumar, N. Hallab, M.A. Wimmer; J. Jacobs, S. Prasad. Electrochemical Biosensor To Detect Implant Derived Metal Ions In A Mice Model: Development And Validation. Transactions Vol. 47: 777; Annual Meeting of the Orthopaedic Research Society, Tampa, FL, 2022
- A. Impergre, A.M. Sfarghiu, N. Bernoud-Hubac, M.A. Wimmer. Comparative Tribological Study Of Three Biomaterials For Interposition Shoulder Arthroplasty. Transactions Vol. 47: 1276; Annual Meeting of the Orthopaedic Research Society, Tampa, FL, 2022
- S.P. Mell, C. Yuh, T. Nagel, S. Chubinskaya, H.J. Lundberg, M.A. Wimmer. Differences In The Superficial Zone Behavior Between Human And Bovine Cartilage - A Finite Element Analysis. Transactions Vol. 47: 1483; Annual Meeting of the Orthopaedic Research Society, Tampa, FL, 2022
- S. Radice, G. Tibbits, H. Beyenal, M.A. Wimmer. Influence Of Hyaluronic Acid From Synovial Fluid On The Corrosion Of CoCrMo-Alloys. Transactions Vol. 47: 1716; Annual Meeting of the Orthopaedic Research Society, Tampa, FL, 2022
- C. Yuh, S. Liu, D. Hall; A. Hakimiyan, M.A. Wimmer, S. Chubinskaya, R. Pourzal. FTIR-I Chemical Mapping Of Articular Cartilage As A Function Of Tissue Degeneration - A Preliminary Study. Transactions Vol. 47: 2124; Annual Meeting of the Orthopaedic Research Society, Tampa, FL, 2022
- C. Beckmann, S. Heermant, A. Wittrock, A. Fischer, M.A. Wimmer, J. Debus. The Effect Of Machined Surface Topography On Wear Particle Formation In A TiAlV-CoCrMo Taper Contact Model. Transactions Vol. 47: 2146; Annual Meeting of the Orthopaedic Research Society, Tampa, FL, 2022
- M.A. Wimmer, J. He, N. Shakoor, C. Ferrigno. Gait Training In The Community: Device Development and Proof of Concept Studies. Transactions, International Society for Osteoarthritis, Berlin, Germany, 2022
- J. He, C. Ferrigno, M.A. Wimmer. Movement Strategies Adopted By Individuals With Knee OA in Response To Pressure-Based Auditory Feedback Gait Retraining. Transactions, International Society for Osteoarthritis, Berlin, Germany, 2022
- C. Yuh, S.P. Mell, T. Nagel, S. Chubinkaya, H.J. Lundberg, M.A. Wimmer. Comparison of Mechanical Properties in the Superficial Zone of Young Bovine and Mature Human Articular Cartilage. Transactions, International Society for Osteoarthritis, Berlin, Germany, 2022
- A. Impergre, M.A. Wimmer, A. Sfarghiu, N. Bernoud-Hubac. Tribological Evaluation of a Pyrolytic Carbon Interposition Implant against Live Cartilage in Comparison With Alumina Ceramic and CoCrMo-Alloy. Abstract, International Society for Technology in Arthroplasty, 33rd Annual Congress, Maui, Hawaii, 2022
- J. Hamilton, A. Markovics, G. Della Fara, A. Impergre, M.A. Wimmer. Use of Electrophoretic Deposition in the Hospital Setting to Coat Medical Implants With Therapeutic Agents to Prevent or Treat Biomaterial Associated Infection. Abstract, International Society for Technology in Arthroplasty, 33rd Annual Congress, Maui, Hawaii, 2022
- M. A.Wimmer, A. Fischer, P. Telouk. Why Surface Topography of Hip Trunnions Matters Under Fretting Corrosion. Abstract, International Society for Technology in Arthroplasty, 33rd Annual Congress, Maui, Hawaii, 2022
- G. Della Fara, M.A. Wimmer, J. Hamilton, A. Markovics. Electrophoretic Deposition of Gentamicin and Chitosan into Titanium Nanotubes to Prevent Periprosthetic Joint Infection. Abstract, International Society for Technology in Arthroplasty, 33rd Annual Congress, Maui, Hawaii, 2022
We gratefully acknowledge funding from philanthropy, industry, foundations, as well as federal agencies, including the National Science Foundation and the National Institutes of Health.
Contact Us
Department of Orthopedics
RUSH University Medical Center
1611 W. Harrison St, Suite 204-205
Chicago, IL 60612
The group is always looking for PhD students and post-doctoral researchers. If you are interested in joining us and would like to find out about any current vacancies please contact Dr. Wimmer (Markus_A_Wimmer@rush.edu). If you are interested in collaborating with us or joining our research team, please get in touch with a relevant staff member.
