
The Robbins and Jacobs Family Biocompatibility and Implant Pathology Laboratory is part of the Department of Orthopedic Surgery at Rush and is located in the Sophija and Jorge O. Galante Orthopedic Building. It is our mission to investigate the different causes and underlying mechanisms of orthopedic implant failure and thereby provide information to the orthopedic research community on how to prevent premature implant failure. The focus of our research lies on the analysis of retrieved tissues and implant devices from patients with joint replacements. Such devices can either be surgically retrieved components from revision surgery (failure cases) or postmortem retrievals (well-functioning devices).

Our work
The main emphasis of our lab has been retrieved joint replacements for the hip, knee, and shoulder, but we also study retrieved ankle replacements and spine implants. The postmortem retrievals are collected by our team according to a previously published protocol. The analysis of retrieved tissue concentrates especially on tissue reactions to foreign bodies such as wear debris and corrosion products that are generated from joint replacements. In order to determine the type of tissue reaction that has occurred, we employ specific histology and immunohistochemical methods. Furthermore, we are able to analyze the chemical and structural composition of foreign bodies using custom developed methods. Our device retrieval analysis includes the analysis of specific damage features and the determination of the occurring damage mode, implant alloy metallurgy, determination of material loss using metrology methods, wear particle analysis and the analysis of the interaction of implant surfaces with the body (e.g., osseointegration). For the latter, we are also able to conduct in vivo studies using established animal models.
Specific areas of research include the following:
- To determine failure modes of failed joint replacements
- Analysis of tissue reactions to wear debris and corrosion products generated from implants
- Systemic reactions to wear debris and corrosion products
- Animal models to test osseointegration properties of implant surfaces and bone graft substitutes
- Determination of material loss from implant surfaces (metrology)
- Implant alloy characterization (metallography)
- Particle analysis
Our team consists of experts in orthopedic science, biology, histology, materials science and engineering, in order to have a coordinated effort in studying the entire patient/implant construct correlating the cellular responses of interface, local and systemic tissues of the body to the wear, corrosion, and metallurgy of the joint components.
-
Faculty
- Deborah J. Hall, assistant professor, director of the retrieval laboratory
- Robin Pourzal, PhD, associate professor, director of implant materials analysis
- Thomas Turner, DVM, associate professor
- Robert M. Urban, professor emeritus, former director
-
Staff
 Bianca Romay, BS, research assistant
Bianca Romay, BS, research assistantBianca received her bachelor’s degree in Biology with emphasis on Medical Sciences from the University of Illinois at Chicago. While completing her undergraduate degree, she joined Dr. Shiva Shahrara’s laboratory in the Division of Rheumatology at UIC as a research assistant. She aided in discovering new targets and biomarkers for RA disease severity and response to effective therapy. Her recent focus is on analyzing the mechanical and chemical effects of implant devices and joint replacements along with their effects on localized tissue. Prior to her bachelor’s degree in Biology, Bianca was studying Kinesiology as a student-athlete while playing soccer at Western Illinois University. In her free time, she continues to play soccer, coach younger teams and train athletes of all ages.
Past experience and research interests include the following: biomechanics, human physiology, rheumatology, histology, immunohistochemistry.
Email ResearchGate Publications
- Jennifer Wright, MS, research assistant
-
Postdoctoral fellowsImage

Mozart Queiroz Neto, PhD
After obtaining bachelor’s degree in Metallurgical Engineering from Universidade Federal do Ceará in Brazil, he initially followed an industrial career as metallurgist in the steelmaking industry. He later joined The University of Sheffield in the United Kingdom where he earned his Ph.D. in Materials Engineering. He has experience in Ti alloys for biomedical use, tribocorrosion and electron microscopy.
Research interests: characterization of implant alloys, failure mechanisms, tribology, corrosion, tribocorrosion and additive manufacturing.Email LinkedIn ResearchGate Publications
Image
Catherine Yuh, PhD
After receiving a bachelor’s degree in Bioengineering from University of Illinois at Urbana Champaign, Catherine pursued a Master’s in Biotechnology from Rush. This experience piqued her interest in research and led her to the Joan and Paul Rubschlager Tribology Laboratory at Rush, where she received her Ph.D. under Dr. Markus Wimmer working on cartilage biotribology, mechanics, and mechanobiology. Now she is a postdoctoral fellow sponsored by a NIH T32 grant. Her current research relies on methods including microindentation and staining-free histology via spectroscopic imaging, to further understand mechanical-biological relationships in musculoskeletal tissue systems and disorders.
Research interests: microindentation, mechanical characterization, spectroscopic imaging, biotribology, biomaterials, soft matter.
-
Graduate studentsImage

Songyun Liu, MS
Songyun received both his B.S. and M.S. degrees in Materials Science and Engineering. Upon graduation on 2016, he first joined Dr. Pourzal’s lab as a research assistant to initiate the endeavor of using advanced spectroscopic imaging (IR, Raman, and hard X-ray) for analysis of biological samples. Specifically, he is studying the implant debris and their biological impact on joint capsule tissue, as well as distal organs. He started his Ph.D. on 2018 (Bioengineering, University of Illinois at Chicago) to continue the same research work. He enjoys playing basketball, hiking, and indoor bouldering.
Research interest: Advanced Spectro-microscopic analysis on ‘implant debris-capsule tissue’ interaction; Spectral-biomarker for specific tissue response; pathology for arthritic joint.
Stephanie McCarthy, BS
Stephanie received her bachelor’s degree in Biology with a focus in anatomy and evolution from Elmhurst University, Elmhurst, IL. After completing her undergraduate degree, she began working in the Implant Pathology and Biocompatibility Lab as a Research Technician, managing day-to-day activities in the lab and providing support to research efforts. She is now a graduate student in the Integrated Biomedical Sciences Doctoral Program at Rush University Graduate College, focusing on altered immune responses to implant debris both locally and systemically.
Research interests: Adverse Local Tissue Reaction classification, histology, immunohistochemistry, microscopy, sample preparation, lymphocyte response (and dinosaurs, in her spare time).
Image
William C. Elzemeyer, BA
Growing up in Jackson Hole, Wyoming allowed William to approach life with a different viewpoint from many of his peers. He graduated from the University of Washington with a bachelor’s degree in Earth and Space Science: Physics Route with a minor in Mathematics. During his time there he was able to work on multiple rocket launches under Robert Winglee’s advanced propulsions lab. After finishing his time in Seattle, he returned to the Rockies where he was initially exposed to working in the medical field with Dr. Joshua Beck at Teton Orthopedics. Dr. Beck took William under his wing as his medical assistant for the better part of three years, finally culminating with William’s return to the academic world as he pursues his master’s degree in biotechnology at Rush University.
Research interests: Adverse Local Tissue Reaction classification, mechanical characterization, spectroscopic imaging.Image
Emily Yee, BA
Emily received her B.A. in Health and Human Physiology from the University of Iowa in 2022. While completing her undergraduate degree, she joined Dr. Yumi Imai's laboratory in the Fraternal Order of Eagles Diabetes Research Center as a research volunteer. She assisted in diabetic research that aims to understand the mechanism behind pancreatic beta cell failure in Type 2 diabetes by focusing on the role of lipid droplet protein, perilipin 5, in insulin secretion through regulation of intracellular lipid metabolism. Her interest in clinical research shifted towards orthopedics and rehabilitation during her internship as a pulmonary rehab aid at the University of Iowa Hospital & Clinics and working in patient care with Vietnam-era amputees and veterans at the VA hospital. Emily is now a PT aide at Rush Physical therapy and a graduate student in the Biotechnology Master Program at Rush University Graduate College, studying histology and morphology of femoral heads with CAM-type FAIS.
Research interest: Immunohistochemistry, histology, spectroscopic imaging, rehabilitation medicine, physiotherapy, outcome measures in physical therapy, implant pathology, PT treatment effectiveness and modalities.
-
Medical students, Undergraduate students, and VolunteersImage

Alexander “Al” Hornung
He is currently a third-year M.D. candidate, NIH T32 grant recipient and recent Carolyn L. Kuckein Student Research Fellowship recipient. His recent research is focused on wear and corrosion in failed total shoulder arthroplasty (TSA) prostheses as well as periprosthetic joint infection. Past experience and research interests include gait analysis, spine, machine learning, and arthroplasty with a recent focus on sports medicine and implant pathology topics pertaining to the shoulder and hip conditions.
Image
Daehan Justin Yi
Justin received his bachelor's degree in Biology from Washington University in St. Louis and is currently an M.D. candidate at Rush Medical College. He is the recipient of the Rush Medical College and Rush Medical College Alumni Association Summer Research Fellowship, and was designated as an Alumni Association Scholar. Justin's research focuses on implant alloy microstructure and corrosion properties of total shoulder arthroplasty (TSA) prostheses. Past research experiences include heme-based trafficking and signaling in sepsis, and immunophenotyping of pro- and hypoinflammatory states to identify timing for immunoadjuvant therapies.
Image
Colton Mowers, BA
Colton received his bachelor’s degree in Genetics with a certificate in Leadership from the University of Wisconsin – Madison and is currently an M.D. candidate class of 2026 at Rush Medical College. Colton’s current research focuses on implant pathology and corrosion properties in failed total shoulder arthroplasty (TSA) prostheses. Future work will also be aimed at novel identification techniques of periprosthetic joint infections in TSAs. Past research includes medial collateral ligament (MCL) repair and construction, along with genetic analysis and manipulation using CRISPR in mouse models.
- Alannah Rodrigues
-
Clinical collaborators
Adult Reconstruction (Hip and Knee)
- Brett R. Levine, MD
- Craig J. Della Valle, MD
- Joshua J. Jacobs, MD, (Chairperson)
Sports Medicine (Shoulder)
Temporomandibular Joint (TMJ) Implants
- Louis G. Mercuri, DDS, MS
-
Former group members
- Mable Je, BS
- Kirsten Sipek, MS
- Mario C. Paolantonio, MS
- Jonas Ehrich, MS
-
Awards
- Songyun Liu, Recipient of a Implant Section Best Podium Award (3rd place) at the ORS 2023 Annual Meeting
- Catherine Yuh, Recipient of a New Investigator Recognition Award (NIRA) at the ORS 2023 Annual meeting
- Yuh C, Recipient of a 2022 AO Spine Research Start-up Grant, AO Spine
- Yuh C, Finalist (Top 3) in the ON/AANA Orthoregeneration Grant Tank Competition 2022, ON Foundation/AANA
- Hall DJ, Recipient of ORS Orthopaedic Implants Section Poster Award (2nd place) at the ORS 2022 Annual Meeting 02/2022
- Yuh C, Best Abstract Award of the 2021 Rush Mentoring Programs 7th Annual Symposium, Rush Mentoring Program
- Liu S, STAR award given by the Orthopedics Special Interest Group at the Society for Biomaterials 2021 Annual Meeting 04/2021
- McCarthy SM, Recipient of ORS Orthopaedic Implants Section Poster Award from ORS 2021 Annual Meeting 02/2021
- Bijukumar DR, William H. Harris, MD Award, awarded by the Orthopedic Research Society (ORS) in 2019. Dr. Bijukumar is a frequent collaborator of our lab at the University of Illinois College of Medicine at Rockford, IL. Dr. R. Pourzal and D.J. Hall are co-authors on this award paper.
-
Newest manuscripts
- Neto MQ, Radice S, Hall DJ, Mathew MT, Mercuri LG, Pourzal R. Alloys Used in Different Temporomandibular Joint Reconstruction Replacement Prostheses Exhibit Variable Microstructures and Electrochemical Properties. Journal of Oral and Maxillofacial Surgery, DOI: https://doi.org/10.1016/j.joms.2021.12.016
- Neto MQ, Radice S, Hall DJ, Nicholas FB, Mathew MT, Fischer A, Jacobs JJ, Pourzal R. Microstructure and Electrochemical Behavior of Contemporary Ti6Al4V Implant Alloys. Journal of Bio- and Tribo-Corrosion, DOI: https://doi.org/10.1007/s40735-021-00623-3
- McCarthy SM, Hall DJ, Mathew MT, Jacobs JJ, Lundberg HJ, Pourzal R. Are damage modes related to microstructure and material loss in severely damaged CoCrMo femoral heads? 2021, DOI: 10.1097/CORR.0000000000001819
- Neto MQ, Yuh C, Van Arkel R, Hall DJ, Espinoza-Orías AA, Pourzal R. The effect of additive manufacturing parameters on microstructure and mechanical properties of Ti-6Al-4V alloy for biomedical applications, ASTM, Special Technical Publications, 2021, DOI: http://doi.org/10.1520/STP163720200121
- Zachariah Z, Liu Z, Balachandran S, Pourzal R, Hall DJ, Fischer A, Raabe D, Herbig M. On the formation mechanism of column damage within modular taper junctions, Journal of Arthroplasty, 2021, DOI: https://doi.org/10.1016/j.arth.2021.02.073
- Liu S, Hall DJ, Della Valle C, Walsh M, Jacobs JJ, Pourzal R. Simultaneous characterization of implant wear and tribocorrosion debris and its corresponding tissue response using infrared chemical imaging, Biotribology, 2021, DOI: https://doi.org/10.1016/j.biotri.2021.100163
- Metha N, Pourzal R, Hall DJ. Garrigues G. Anatomic Shoulder Arthroplasty Biomaterials: Their Features, Function and Effect on Outcomes. Journal of Bone and Joint Surgery Reviews, 2020, Volume 9, Issue 9 – p e19.00212, DOI: 10.2106/JBJS.RVW.19.00212
-
Upcoming presentations
- Conferences
-
Recent presentations
- Conferences:
- Liu S, McCarthy SM, Hall DJ, Jacobs JJ, Pourzal R. Is the Wear of the Polyethylene Liner Still a Problem: A Study of Macrophage Chemistry Using HD-FTIR Imaging, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 72 (podium)
- Yuh C, Malloy P, Mell SP, Jackson G, Chahla J, Pourzal R, Hall DJ, The Relationship Between Disease Severity and Tissue Expression in the Cam Lesion and Capsule Tissues Retrieved from Patients Hips With Cam-Type Femoroacetabular Impingement Syndrome, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 218 (podium, NIRA award nominee)
- Rathjen JL, Mell SP, Pourzal R, Levine BR, Gustafson JA, Lundberg HJ, The Effects of Assembly Force Magnitude on Total Hip Replacement Modular Junction Contact Mechanics, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 56 (podium)
- Neto MQ, Wright JL, Romay B, Yuh C, Hall DJ, Rainforth WM, Jacobs JJ, Pourzal R, Retrieval Analysis and Effect of Grain Size on the Corrosion Behavior on he Occurrence of Gross Trunnion Failure of TMZF Implant Alloy, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 386 (poster)
- Samelko L, McAllister K, Jacobs JJ, Pourzal R, Hallab NJ, Comparative Immune and Neurotoxicity Effects of Orthopedic Implant Metal Debris, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 376 (poster)
- Hall DJ, Yuh C, Neto MQ, Mercuri LG, Cordero KE, Pourzal R, Retrieval Analysis of In Vivo Wear of Temporal Mandibular Joint Replacement Prostheses of One Design, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 397 (poster)
- Travers N, Cheng K, Orias AE, Pourzal R, Arkel RV, Mathew M, Comparative Tribocorrosion Assessment of 3D Printed and Commercial Ti Alloys: In Vitro Hip Simulator Testing, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 812 (poster)
- Sun Y, Kanniyappan H, Ramachandran RA, Neto MQ, McNallan M, Pourzal R, Lundberg H, Mathew M, Simulating the Low Magnitude Fretting-Corrosion at Hip Implant Taper Junctions: An In-Vitro Model, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 825 (poster)
- McCarthy SM, Samelko L, Hall DJ, Pourzal R, Hallab NJ, A Novel Use of the Particle Induced Osteolysis Murine Model to Study CoCrMo Trafficking to Brain Tissue, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 1201 (poster)
- Godoy M, Sipek K, Gustafson J, Levine B, Pourzal R, Lundberg H, Surgical Impaction Force in Benchtop Head-Stem THA: Effect of Surgeon Experience and Assembly Technique, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 1652 (poster)
- Liu S, Hall DJ, Chee AV, Aboushaala K, Yuh C, Oh C, Zehra U, Orias AE, An H, Pourzal R, Samartzis D, Calcium Pyrophosphate Dihydrate Crystal Deposition (Pseudogout) Develops in Symptomatic Degenerated Intervertebral Discs of Patients, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 1735 (poster)
- Chee AV, Liu S, Yuh C, Pourzal R, Moran M, Naqib A, Green S, Williams B, Persons AL, Napier TC, Samartzis D, An HS, Endplate Injury Induces Disc Degeneration, Gut Microbiome and Brain Changes, and Back Pain in the Rat Model, ORS 2023 Annual Meeting, Dallas, TX, February 10-14, 2022: Paper No. 1833 (poster)
- Hall DJ, Pourzal R. The Role of Design, Microstructure, and Malseating in the Fretting and Corrosion of Dual Mobility Hip Metal Liners, Annual Meeting of the International Society for Technology in Arthroplasty (ISTA 2022), Maui, Hawaii, Aug 31- Sept 3 2022
- Hall DJ, Turner TM, McCarthy SM, Wright J, Urban RM, Pourzal R. Successful Long-Term Bone Ingrowth and Minimal Polyethylene Wear of Bilateral MOP THA in a Lowland Gorilla Compared to Human Postmortem Retrievals, Annual Meeting of the International Society for Technology in Arthroplasty (ISTA 2022), Maui, Hawaii, Aug 31- Sept 3 2022
- Hall DJ, McCarthy SM, Wright J, Jacobs JJ, Pourzal R. MOP Patients With High ALVAL Tissue Scores Had CoCrMo Femoral Heads Exhibiting a Chemical Attack of Preferential Corrosion Sites, Annual Meeting of the International Society for Technology in Arthroplasty (ISTA 2022), Maui, Hawaii, Aug 31- Sept 3 2022
- McCarthy SM, Hall DJ, Mell SP, Levine BR, Jacobs JJ, Pourzal R. Has Wrought CoCrMo Alloy Changed for the Worse Over Time? ISTA 33rd Annual Congress: 7759, 2022. Accepted for podium presentation
- McCarthy SM, Neto MQ, Hall DJ, Jacobs JJ, Pourzal R. Chemically Dominated Corrosion Modes in CoCrMo and Ti-Alloy Femoral Stem Tapers, ISTA 33rd Annual Congress: 7806, 2022. Accepted for e-poster presentation
- Neto MQ, Wright JL, Romay B, Hall DJ, Rainforth WM, Jacobs JJ, Pourzal R. Does TMZF Alloy Microstructure Play a Role in Corrosion Behavior and the Occurrence of Gross Trunnion Failure? ISTA 2022, Maui, Hawaii, Aug 31- Sept 3 2022
- Neto MQ, Frisch N, Hall DJ, Levine BR, Jacobs JJ, Pourzal R. Intra-Manufacturer Microstructural Variations of Additively Manufactured Implants and Its Implications on Corrosion Behavior, ISTA 2022, Maui, Hawaii, Aug 31- Sept 3 2022
- Yuh C, Burnett R, Rodrigues A, Hall DJ, Nam D, Pourzal R. In Vitro Comparison of High and Low Viscosity Cement 'Lack of Bonding' at the Cement-Implant Interface in Total Knee Arthroplasty (ISTA 2022), Maui, Hawaii, Aug 31- Sept 3 2022. Accepted for podium presentation
- Yi DJ, Hornung A, Neto MQ, Hall DJ, Romay B, Nicholson G, Garrigues GE, Pourzal R. In Vitro and in Vivo Corrosion Behavior of CoCrNiFeMo Alloy in Total Shoulder Arthroplasty, Annual Meeting of the International Society for Technology in Arthroplasty (ISTA 2022), Maui, Hawaii, Aug 31- Sept 3 2022
- Neto MQ, Pourzal R. Microstructural and Electrochemical Characterization of 3D Printed Biomedical Implants. International Conference on Metallurgical Coatings and Thin Films (ICMCTF) , San Diego, California, USA, from May 22-27 2022
- Neto MQ, Hall DJ, Jacobs JJ, Pourzal R. Microstructure and Electrochemical Behavior of Conventional and Additively Manufactured Orthopedic Components Made of Ti6Al4V. Corrosion in 4D
- Hornung AL, Hall DJ, Nicholson GP, Pourzal R, Garrigues GE, Association of C. Acnes with Histopathologic and Tribological Characteristics of Retrieved Total Shoulder Arthroplasties. Annual Meeting of the American Academy of Orthopedic Surgeons (AAOS), March 22-26, Chicago: P0101
- Hall DJ, McCarthy SM, Wright JL, Jacobs JJ, Pourzal R. Femoral Heads with Column Damage Exhibit Higher ALVAL Scores Than Those with Fretting-Corrosion Only. Annual Meeting of the American Academy of Orthopedic Surgeons (AAOS), March 22-26, Chicago: P0570
- Terhune EB, Serino J, Pourzal R, Hall DJ, Nam D, Jacobs JJ, Della Valle CJ. Radiographic and Corrosion Analysis of Modular Dual-Mobility Liners in Total Hip. Annual Meeting of the American Academy of Orthopedic Surgeons (AAOS), March 22-26, Chicago: P0524
-
Mehta N, Perry AK, Knapik DM, Hall DJ, Clapp IM, Garrigues GE, Verma NN. A Histological Analysis of the Superior Capsule of the Glenohumeral Joint and the Rotator Cuff, Rush Surgical Society Meeting, March 16, 3rd place winner of 2022 Rush Resident Research Competition
-
Hall DJ, Terhune EB, Serino J, Nam D, Jacobs JJ, Della Valle CJ, Pourzal R. Fretting And Corrosion Of Dual Mobility Hip Metal Liners: Role Of Design, Microstructure, And Malseating. ORS 2022 Annual Meeting. February 4-8 2022
-
McCarthy SM, Hall DJ, Wright JL, Jacobs JJ, Lundberg HL, Pourzal R. Role of CoCrMo Alloy Banding on the Corrosion Behavior of Postmortem Retrieved Femoral Heads. Trans ORS 47: 1731, 2022
-
Yi DJ, Neto MQ, Hall DJ, Romay B, Wright JL, Radice S, Nicholson G, Garrigues GE, Pourzal R. Corrosion behavior of implant alloys used in total shoulder arthroplasties. ORS 2022 Annual Meeting. February 2022
-
Neto MQ, Radice S, Hall DJ, Jacobs JJ, Mathew MT, Pourzal R. The Electrochemical Properties of Ti-6Al-4V Orthopaedic Implants Relies on the Alloy's Microstructure and Features. ORS - Orthopaedic Implants Virtual Scientific Session. September 29th
-
Neto MQ, Mercuri L, Pourzal R. Microstructural Characterization and its Effect of Electrochemical Behavior of TMJ Implants. Biomedical Implants Session, SIBF BioInterface 2021. September 2021
-
Neto MQ, Electrochemical Behavior of Conventional and Additively Manufactured Ti6Al4V Alloy Implant Components. The ASTM International Conference on Additive Manufacturing (ASTM ICAM 2021). November 1-5, 2021. JW Marriott - Anaheim, CA, USA | Virtual
- Pourzal R, Characterization of Intra-Cellular Metallic Debris from Total Hip Replacements within Periprosthetic Tissue, APS/CNM Users Meeting 2021, Argonne National Laboratories, Lemont, IL, May 13th 2021
- McCarthy SM, Neto MQ, Je M, Hall DJ, Jacobs JJ, Pourzal R. CoCrMo Alloy Features Affecting Material Loss in Severely Damaged THA Femoral Head Tapers. Society for Biomaterials (SFB) 2021 Annual Meeting. April 2021
- Neto MQ, Radice S, Hall DJ, Jacobs JJ, Mathew MT, Pourzal R. Does Microstructure Influence the Corrosion Behavior of Ti-6Al-4V Orthopedic Implants? Society for Biomaterials (SFB) 2021 Annual Meeting. April 2021
- Liu S, Hall DJ, McCarthy SM, Jacobs JJ, Pourzal R. Chemical Imaging of Pseudo-capsule Macrophages in Response To Metal Debris: A Group Comparison of MoM HR vs. MoP THR. STAR award from Society for Biomaterials (SFB) 2021 Annual Meeting. April 2021 (podium)
- Liu S, Hall DJ, McCarthy SM, Jacobs JJ, Pourzal R. Characterization of Chemical Alterations Within Pseudo-capsule Macrophages in Response to Metal Debris. ORS 2021 Annual Meeting. February 15th 2021 (podium)
- Pourzal R. Lessons from Retrieval Implants: Differences in Addictive vs. Conventional Manufactured Metallurgy. ORS 2021 Annual Meeting. February 15th 2021
- Hall DJ. Lymphocyte-dominated Adverse Local Tissue Reactions are Associated with a Chemical Attack of Preferential Corrosion Sites of CoCrMo Heads in MoP THA. ORS Annual Meeting. February 15th 2021
- Pourzal R. Could Alzheimer's Disease Pathology be Associated with Metals Released from Total Joint Replacements? ORS 2021 Annual Meeting. February 14th 2021
- Hornung A, Hall DJ, Je M, Wright S, Nicholson G, Garrigues G, Pourzal R. Do Total Shoulder Arthroplasty Implants Corrode? Oral presentation at ORS 2021 Annual Meeting. February 14th 2021.
- McCarthy SM, Neto MQ, Je M, Hall DJ, Jacobs JJ, Pourzal R. Effects of Alloy Microstructure on Material Loss in Severely Damaged CoCrMo THA Femoral Head Tapers. ORS 2021 Annual Meeting. February 13th 2021
- Neto MQ, Radice S, Hall DJ, Jacobs JJ, Mathew MT, Pourzal R. Effect of Microstructure on the Corrosion Behaviour of Ti-6Al-4V Orthopaedic Implants. ORS 2021 Annual Meeting. February 13th 2021
- Neto MQ, Hall DJ, Frisch NB, Fischer A, Jacobs JJ, Pourzal R. Is Ti-6Al-4V Alloy Always the Same in Orthopaedic Implants? ISTA's New Early-career Webinar Series. November 20th 2020
- Neto MQ, Hall DJ, Frisch NB, Fischer A, Jacobs JJ, Pourzal R. Microstructural Comparison of Additive Manufactured and Conventional Total Joint Replacement Components Made from Titanium Alloy. ASTM International Conference on Additive Manufacturing (ASTM ICAM 2020). November 19th 2020
- Neto MQ, Arkel RV, Hall DJ, Espinoza A, Pourzal R. The Effect of Additive Manufacturing Parameters on Ti-6Al-4V Alloy Microstructure for Biomedical Applications. ASTM International Conference on Additive Manufacturing (ASTM ICAM 2020). November 19th 2020
- Neto MQ, Hall DJ, Pourzal R. Microstructural Comparison of Additive Manufactured and Conventional Implants Components made of Ti6Al4V. The Rush Mentoring Programs - 6th Annual Symposium. Chicago IL, USA. September 9th 2020
- Neto MQ. Institute of Biomaterials, Tribocorrosion, Nano and Regenerative Medicine (IBTN) monthly seminar (IBTN-US). EBSD for Implant Analysis. Invited speaker. Chicago IL, USA. August 19th 2020
- McCarthy SM, Hall DJ, Wright JL, Levine BR, Lundberg HJ, Pourzal R. Assessment of Material Loss, Damage Modes, and Microstructure of Severely Damaged THA Femoral Head Tapers. 2020 ORS Annual Meeting; Phoenix, AZ. February 2020 (poster)
- Liu S, Chen S, Hall DJ, Lai B, McCarthy SM, Pourzal R. Speciation Analysis of Intra-cellular Metallic Implant Debris Using Synchrotron XRF-imaging. 2020 ORS Annual Meeting; Phoenix, AZ. February 2020 (poster)
- Liu S, Hall DJ, McCarthy SM, Chen S, Jacobs JJ, Urban R, Pourzal R. Characterization of Wear and Corrosion Product Using Multivariate FTIR-I Analysis. 2019 ISTA 32nd Annual Congress; Toronto, Canada. October 2019 (Young Investigator Award, podium presentation)
- Liu S, Hall DJ, McCarthy SM, Jacobs JJ, Pourzal R. Multi-modal Imaging Analysis on Joint Capsule Tissue from Total Hip Replacement Patient. FACSS-SciX 2019 Conference, Palm Spring, CA, USA. October 2019 (Invited podium presentation)
- McCarthy SM, Kearns SM, Hall DJ, Quigley L, Levine BR, Pourzal R, Lundberg HJ. Femoral Offset and Topographical Geometry are More Important Determinants of Taper Damage in Total Hip Modular Junctions than Flexural Rigidity. 2019 ORS Annual Meeting. February 2019 (oral presentation)
- Conferences:
Our laboratory houses all equipment needed to perform plastic embedded histology, histopathological evaluation of periprosthetic and remote organ tissue, implant failure analysis, and metallography (sample preparation of metallic samples). The following specialized equipment is featured in our laboratory or used for our work:
-
OrthoLux by RedLux
OrthoLux by RedLux (Romsey, UK): This 5-axis optical coordinate measuring machine utilizes a white light confocal confocal sensor to scan implant surfaces. The resulting metrology data can then be utilized to reconstruct surface in 3D and compute material loss due to wear and corrosion. This novel instrument allows for the fast measurement of large implant surfaces (for example, a femoral head with 44m diameter can be scanned at a high resolution in less than 7 minutes). Besides volumetric loss, the data provides also heat maps for the visualization of areas of material loss, and intensity maps that provide a photo realistic representation of the surface without the “mirror” effects.
-
Jeol Field Emission Scanning Electron Microscope
A Field Emission Scanning Electron Microscope (IT500HR, JEOL): This scanning electron microscope (SEM) is equipped with an Energy Dispersive X-ray Spectroscopy (EDS) detector (Ultim Max, Oxford) for the determination of the chemical composition of materials as well as an Electron Backscatter Diffraction (EBSD) detector (C-nano, Oxford Inc.) for the crystallographic characterization of materials. The SEM is an important component of our day-to-day research efforts and is used across all our research projects. For accurate EBSD measurements, sample preparation is key. We therefore use Cross-Section polisher (IB-19530 CP, JEOL) for the final surface treatment after standard metallographic preparation. This device enables optimal surface conditions for EBSD, but also target preparation for subsurface analysis of specific damage features on retrieved implants.
-
Hysitron TI 950 Nanoindenter
Hysitron TI 950 Nanoindenter (Romsey, UK): This nanoindentation (housed in the Tribology Lab) device houses a highly sensitive transducer that is capable of performing indentation at small depths, allowing for the mechanical characterization of material properties at as small as the nanoscale. This is done by indenting a sample material with an indenter probe and finding the relationship between the applied force and the probe displacement. Nanoindentation is a commonly used technique to measure the hardness and stiffness of materials at these small scales, so we can better understand structure-function-performance relationships in materials. In addition to indentation, the Hysitron TI 950 is also capable of performing scratch testing, which allows the measurement of the deformation and fracture properties for materials such as thin films and coatings. Both indents and scratches can be visualized with the TI 950’s scanning probe microscopy (SPM) imaging technique. Between our laboratory and the Tribology Laboratory, this device has been used to assess artificial implant materials (including titanium, oxide ceramics, UHMWPE, and PVA hydrogels), as well as biological materials (musculoskeletal tissues including cartilage and bone).
Image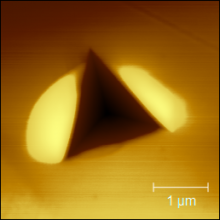 Image
Image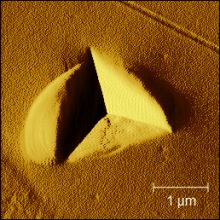
Above: SPM imaging was performed on a TI64 sample following indentation using a Berkovich pyramid tip. On the left, the topography of an area including an indent is visualized, whereas in the right image, the gradient (force) is shown.
-
Fourier Transform Infrared (FTIR) imaging (Agilent)
Fourier Transform Infrared (FTIR) imaging is a well-established analytical method for obtaining spectral and spatial information simultaneously in the micron-size domain. It provides molecular information based on vibrations of present functional groups, which provide a ‘fingerprint’ to identify tissue constituents and underlying biochemical alterations. This approach adds additional chemical information that allows the augmented histopathological analysis for better understanding the implant pathology. Our group is equipped with the Agilent Cary 620 FTIR microscope was coupled to an Agilent Cary 670 FTIR spectrometer (located in the Tribology Lab). It features a large liquid nitrogen cooled MCT focal-plane-array (FPA) detector with 128×128 pixels to image a FOV of 700×700 µm while maintaining the pixel size at 5.5×5.5 µm2 with the standard 15x objective. A high magnification mode with a x5 addition is available within the microscope, which provides a projected pixel size down to 1.1×1.1 µm2. Tissue samples are prepared following conventional histology practice except for using IR transparent slides (usually BaF2, or CaF2) as substrates. The illustration below highlights some of the work from our group.
Image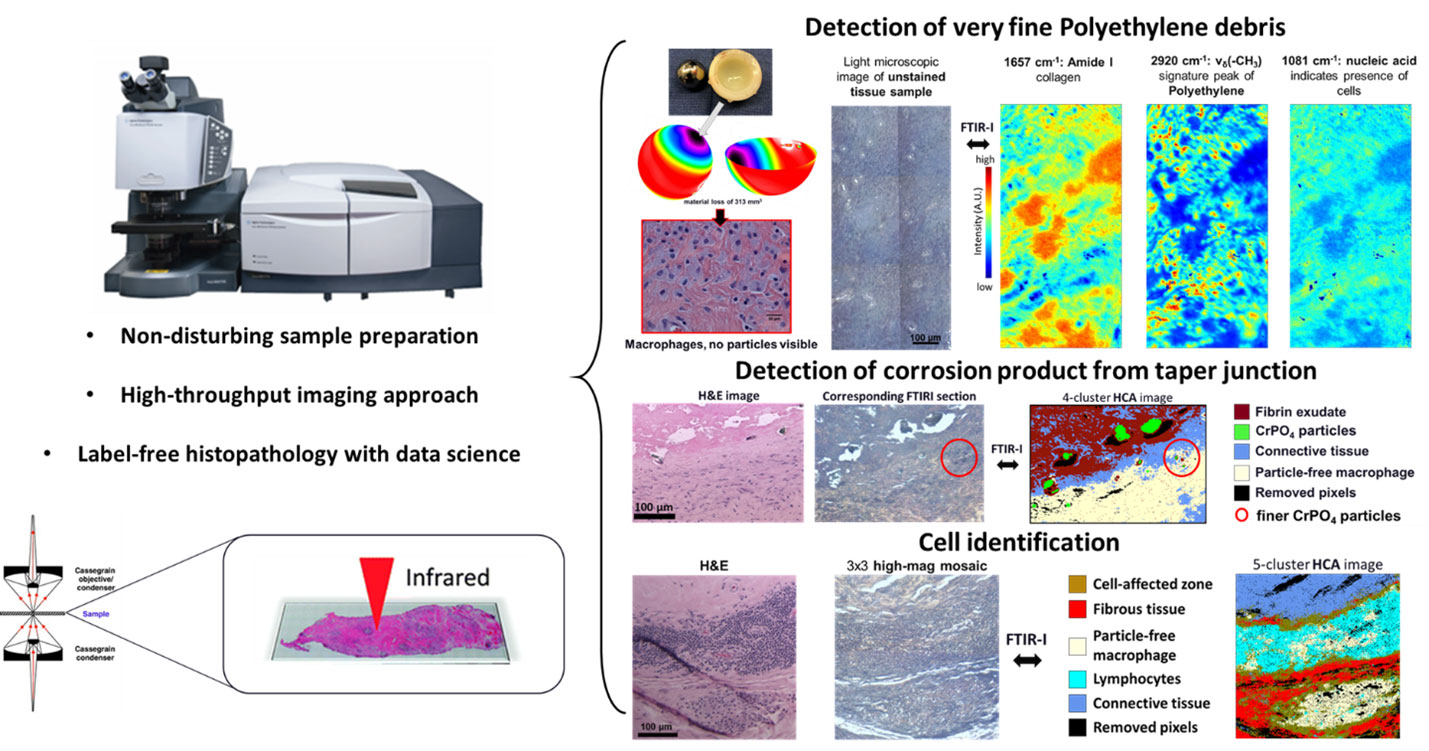
Funding
- NIH/3R01AR070181-05S1, Title: Dissemination of metallic implant debris to the brain and implications on Alzheimer’s disease, (PIs: Pourzal, Hallab)
- NIH/NIAMS R01 AR070181, Title: Corrosion Induced Hip Implant Failure: Synergistic Interaction of Patient, Material, Design, and Surgical Factors, (PIs: Hannah J. Lundberg, PhD, Mathew T. Mathew, PhD, Robin Pourzal, PhD)
- Academy of Shoulder and Elbow Surgeons (ASES) Pilot Grant, Title: “Periprosthetic tissue response to wear and corrosion debris from total shoulder arthroplasties”, (PI: Grant Garrigues, MD)
- NIH/NIBIB R21 EB024039, Title: Determination of excessive immune reactivity to real time implant debris, (PIs: Markus A. Wimmer. PhD, Nadim J. Hallab, PhD)
- Cohn Family foundation/ Rush Mentoring office, Title: Spectroscopic Imaging of Periprosthetic and Remote Organ Tissue of Joint Replacement Patients, (PI: Pourzal)
- Rush Translational Science Consortium (RTSC) Pilot Grant, Title: Corrosion in modular hip implants: Identifying the ideal alloy microstructure, (PI: Pourzal)
Services
- Histopathological analysis of periprosthetic tissues including immunohistochemistry (IHC)
- Implant failure analysis and damage evaluation
- Quantitative wear assessment of surgically or postmortem retrieved as well as in vitro tested implant components by means of an optical co-ordinate measuring machine (CMM)
- Evaluation of bone in-growth via undecalcified embedding and point-counting
- Animal models for the evaluation of synthetic bone graft substitutes and implant biocompatibility
- Metallography for implant alloys
- Advanced microstructural evaluation of implant alloys via electron backscatter diffraction (EBSD) including detailed analysis of manufacturing defects in additively manufactured—3D printed—implant components
- Assessment of particle size using field emission scanning electron microscopy (FE-SEM)
- Chemical analysis of particles within periprosthetic or remote organ tissues by means of Fourier Transform Infrared Spectroscopy Imaging (FTIR-I) and FE-SEM coupled with energy dispersive x-ray spectroscopy (EDS) mapping.
- Chemical analysis of bio-/tribofilms on implant surfaces and structural changes of UHMWPE and PEEK implant components by means of FTIR and Raman spectroscopy.
Publications
Links to PubMed bibliography of laboratory publications:
Contact us
Deborah J. Hall / Robin Pourzal
Department of Orthopedic Surgery
Rush University Medical Center
1611 W. Harrison Street, Suite 207
Chicago, IL 60612
Phone: 312-942-3217
Fax: 312-942-2101
