Amber Lab
Research Mission
As a translational research lab, the fundamental goal of the Amber Lab is to better understand why autoimmune blistering diseases occur and apply this towards improving diagnosis and treatment. The Amber lab uses cutting edge techniques such as single cell RNA-sequencing, CITE-seq, spatial transcriptomics, proteomics, and other large-scale analyses to better understand biologic processes at a more global scale.
Main Projects
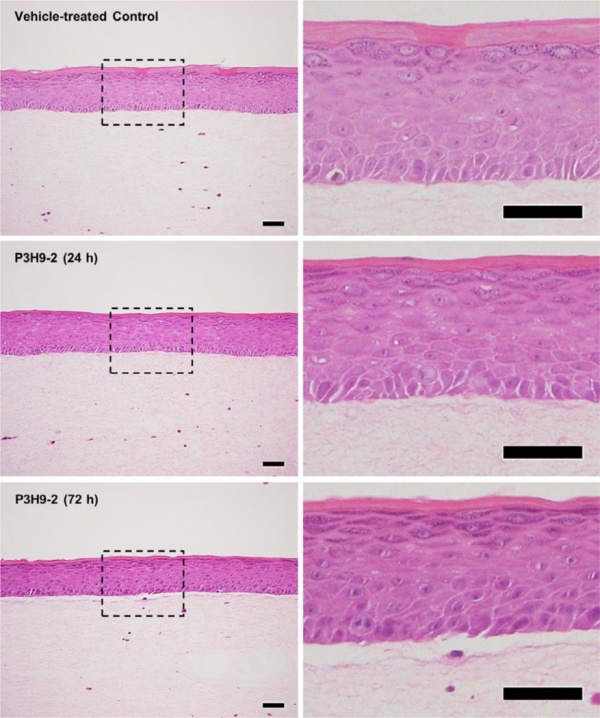
- Local Immune Response in Pemphigoid: The biology of pemphigoid diseases can be simplified to the development of antibodies which bind to proteins providing structural support to epithelial cells. This results in blistering and immune cell recruitment. Our lab seeks to understand how these antibodies directly affect epithelium, and how keratinocytes interact with immune cells. We are particularly interested in the effect of neutrophils and eosinophils.
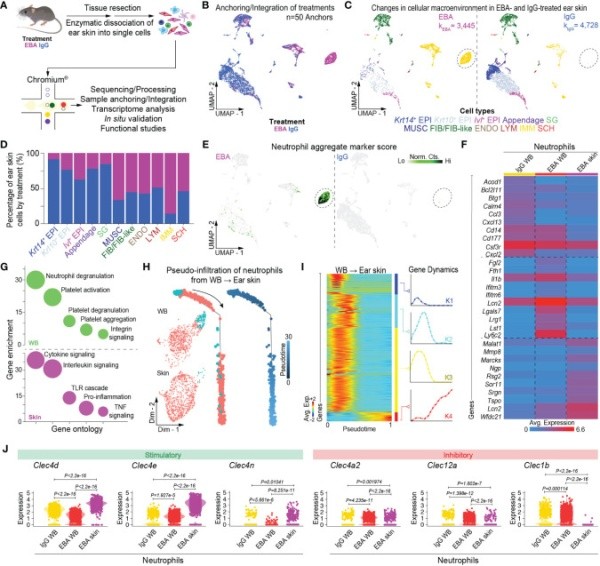
- Granulocyte Biology in Pemphigoid: We are interested in how granulocytes change phenotype as they go from the blood to the skin. We have developed a novel single cell RNA-sequencing protocol which allows capture of viable skin infiltrating granulocytes. This has provided novel insight into the pathogenic role of granulocytes, as well as their phenotypic plasticity.
- Targeting Antigen Specific B-cells: We have developed several novel therapies to target antigen-specific B-cells. We additionally utilize CITE-seq and spatial CITE-seq to reverse engineer therapeutic mechanisms of IVIg to develop more targeted therapies.
- Improving the Diagnosis and Treatment of Ocular Pemphigoid: Currently, the diagnosis of ocular pemphigoid is severely limited by poor sensitivity of blood assays, as well as limited sensitivity of conjunctival biopsy. Conjunctival biopsy is likewise highly invasive. Our lab is developing a highly sensitive blood test to diagnose ocular pemphigoid and other autoimmune blistering diseases in a single assay.
Key Publications
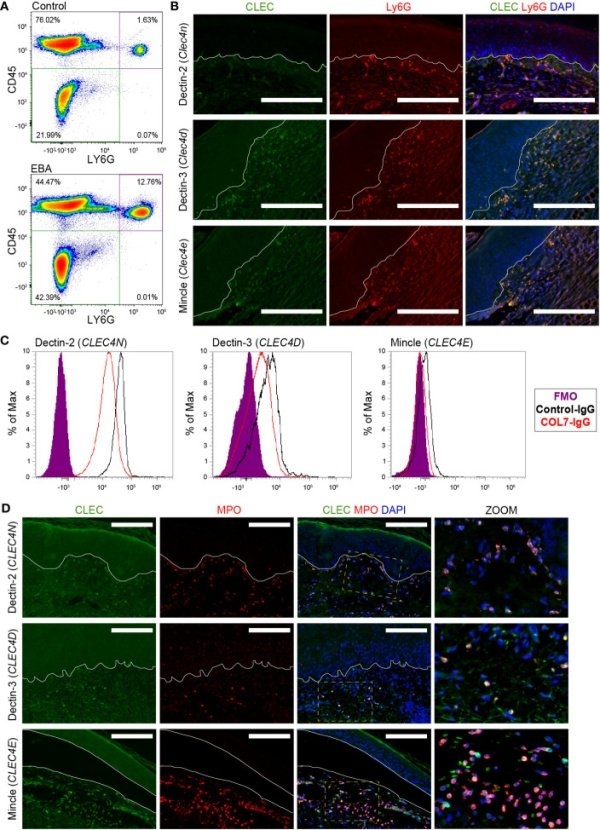
Guerrero-Juarez CF, Schilf P, Li J, Zappia MP, Bao L, Patel PM, Gieseler-Tillmann J, Murthy S, Cole C, Sverdlov M, Frolov MV, Hashimoto T, Ishii N, Rülicke T, Bieber K, Ludwig RJ, Sadik CD, Amber KT. C-type lectin receptor expression is a hallmark of neutrophils infiltrating the skin in epidermolysis bullosa acquisita. Front Immunol. 2023 Sep 20;14:1266359.
Cole C, Vinay K, Borradori L, Amber KT. Insights Into the Pathogenesis of Bullous Pemphigoid: The Role of Complement-Independent Mechanisms. Front Immunol. 2022 Jul 7;13:912876
Bao L, Li J, Solimani F, Didona D, Patel PM, Li X, Qian H, Ishii N, Hashimoto T, Hertl M, Amber KT. Subunit-Specific Reactivity of Autoantibodies Against Laminin-332 Reveals Direct Inflammatory Mechanisms on Keratinocytes. Front Immunol. 2021 Nov 25;12:775412.
Bao L, Perez White BE, Li J, Patel PM, Amber KT. Gene expression profiling of laminin α3-blocked keratinocytes reveals an immune-independent mechanism of blistering. Exp Dermatol. 2022 Apr;31(4):615-621.
Solimani F, Didona D, Li J, Bao L, Patel PM, Gasparini G, Kridin K, Cozzani E, Hertl M, Amber KT. Characterizing the proteome of bullous pemphigoid blister fluid utilizing tandem mass tag labeling coupled with LC-MS/MS. Arch Dermatol Res. 2022 Nov;314(9):921-928.
Bao L, Li J, Perez White BE, Patel PM, Amber KT. Inhibition of dipeptidyl-peptidase 4 induces upregulation of the late cornified envelope cluster in keratinocytes. Arch Dermatol Res. 2022 Nov;314(9):909-915.
Extramural sources, including the National Institutes of Health as well as industry and philanthropic sources, support the Amber lab. As the National Institutes of Health funds less than 20% of applications a year, philanthropic support has become more critical than ever to keep research programs alive, particularly in rare diseases. If you would like to help champion the Amber lab’s work improving the diagnosis and treatment of autoimmune blistering diseases by making a philanthropic donation, please email Charles Palys, director of major gifts, at charles_palys@rush.edu or Dr. Amber at kyle_amber@rush.edu. Your gift — of any amount — can help improve health outcomes for all.
