Departmental Research
Biomaterials Laboratory
Chicago Artificial Musculoskeletal Intelligence Lab
Computational Biomechanics Laboratory
Functional Orthopedics Research and Modeling Laboratory
International Spine Research & Innovation Initiative
Joan and Paul Rubschlager Tribology Laboratory
Motion Analysis Lab
Rush-IBTS Research Fellowship Program
Spine Biomechanics Laboratory
Sports Medicine Research Laboratory
The Robbins and Jacobs Family Biocompatibility and Implant Pathology Laboratory
Laboratory of Adam B. Wilson, PhD
Laboratory of Amarjit S. Virdi, PhD
Laboratory of Carl Maki, PhD
Laboratory of Christopher Ferrigno, PhD, PT
Laboratory of D. Rick Sumner, PhD
Laboratory of Frank Ko, PhD
Laboratory of Jeffrey A. Borgia, PhD
Laboratory of Jitesh Pratap, PhD
Laboratory of Joan A. O’Keefe, PhD
Laboratory of Lei Duan, MD
Laboratory of Meghan Moran, PhD
Laboratory of Ryan Ross, PhD
Cardiology Research
Endocrinology and Metabolism Research
General Internal Medicine Research
Geriatrics, Gerontology and Palliative Medicine Research
Hematology, Oncology and Stem Cell Transplant Research
Hospital Medicine Research
Infectious Diseases Research
Nephrology Research
Preclinical Catheterization Laboratory
Pulmonary Medicine, Critical Care & Sleep Medicine Research
Rheumatology Research
Allergy & Immunology Research
Cardiology Research
Critical Care Research
Developmental/Behavioral Research
General Pediatrics Research
Hematology/Oncology Research
Infectious Disease Research
Neonatology Research
Neurology Research
Psychology Research
Pulmonary Research
Laboratory of Elizabeth Berry-Kravis, MD, PhD
Laboratory of Narine Hakobyan, PhD
Rush NICU Human Milk Research Program
Laboratory of Thomas R. Shannon, DVM, PhD
Laboratory of Lothar A. Blatter, MD, Dr. med.
Laboratory of Fredric Cohen, PhD
Laboratory of Tom DeCoursey, PhD
Laboratory of Robert Eisenberg, PhD
Laboratory of Michael Fill, PhD
Laboratory of Dirk Gillespie, PhD
Laboratory of Joel Michael, PhD
Laboratory of Josefina Ramos-Franco, MD, PhD
Laboratory of Eduardo Ríos, PhD
Autism Assessment, Research, Treatment and Services Center
Center for Mood, Anxiety, and Traumatic Stress Research
Center for Compulsive Behavior and Addiction
Cognitive and Addiction Neuroscience Lab
Road Home Program Research
Treatment Response, Efficacy, Accessibility, and Timing (TREAT) Lab
Woman's Board Depression Treatment Research Center
Departmental Research
Biomaterials Laboratory
Chicago Artificial Musculoskeletal Intelligence Lab
Computational Biomechanics Laboratory
Functional Orthopedics Research and Modeling Laboratory
International Spine Research & Innovation Initiative
Joan and Paul Rubschlager Tribology Laboratory
Motion Analysis Lab
Rush-IBTS Research Fellowship Program
Spine Biomechanics Laboratory
Sports Medicine Research Laboratory
The Robbins and Jacobs Family Biocompatibility and Implant Pathology Laboratory
Laboratory of Adam B. Wilson, PhD
Laboratory of Amarjit S. Virdi, PhD
Laboratory of Carl Maki, PhD
Laboratory of Christopher Ferrigno, PhD, PT
Laboratory of D. Rick Sumner, PhD
Laboratory of Frank Ko, PhD
Laboratory of Jeffrey A. Borgia, PhD
Laboratory of Jitesh Pratap, PhD
Laboratory of Joan A. O’Keefe, PhD
Laboratory of Lei Duan, MD
Laboratory of Meghan Moran, PhD
Laboratory of Ryan Ross, PhD
Cardiology Research
Endocrinology and Metabolism Research
General Internal Medicine Research
Geriatrics, Gerontology and Palliative Medicine Research
Hematology, Oncology and Stem Cell Transplant Research
Hospital Medicine Research
Infectious Diseases Research
Nephrology Research
Preclinical Catheterization Laboratory
Pulmonary Medicine, Critical Care & Sleep Medicine Research
Rheumatology Research
Allergy & Immunology Research
Cardiology Research
Critical Care Research
Developmental/Behavioral Research
General Pediatrics Research
Hematology/Oncology Research
Infectious Disease Research
Neonatology Research
Neurology Research
Psychology Research
Pulmonary Research
Laboratory of Elizabeth Berry-Kravis, MD, PhD
Laboratory of Narine Hakobyan, PhD
Rush NICU Human Milk Research Program
Laboratory of Thomas R. Shannon, DVM, PhD
Laboratory of Lothar A. Blatter, MD, Dr. med.
Laboratory of Fredric Cohen, PhD
Laboratory of Tom DeCoursey, PhD
Laboratory of Robert Eisenberg, PhD
Laboratory of Michael Fill, PhD
Laboratory of Dirk Gillespie, PhD
Laboratory of Joel Michael, PhD
Laboratory of Josefina Ramos-Franco, MD, PhD
Laboratory of Eduardo Ríos, PhD
Autism Assessment, Research, Treatment and Services Center
Center for Mood, Anxiety, and Traumatic Stress Research
Center for Compulsive Behavior and Addiction
Cognitive and Addiction Neuroscience Lab
Road Home Program Research
Treatment Response, Efficacy, Accessibility, and Timing (TREAT) Lab
Woman's Board Depression Treatment Research Center
Image
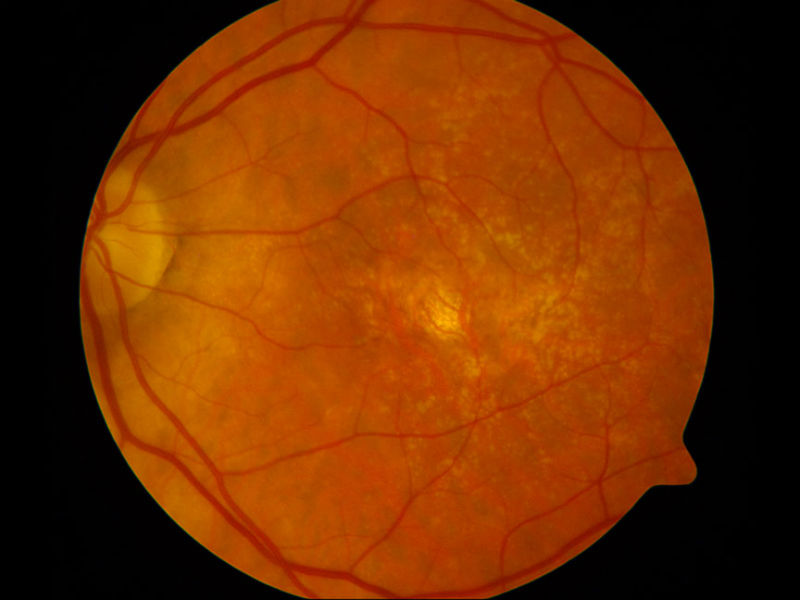
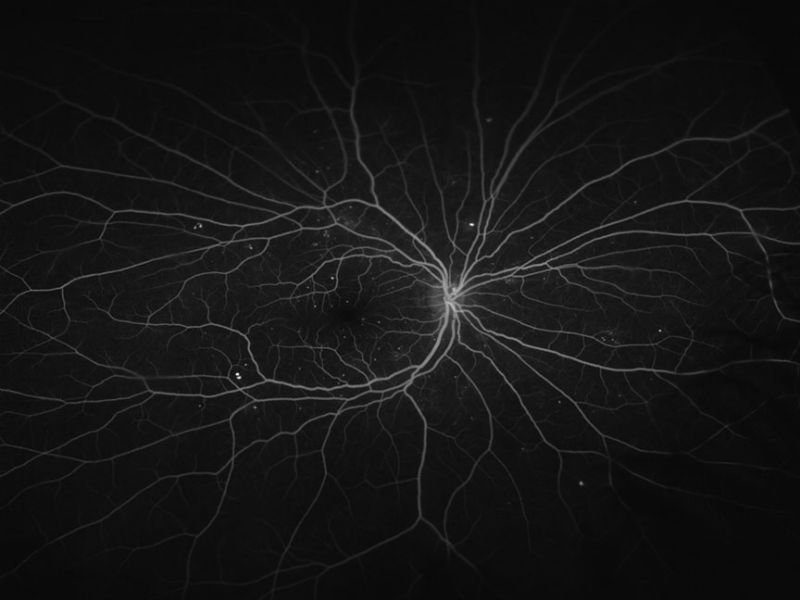
The Department of Ophthalmology is deeply committed to the advance of ophthalmic knowledge and the development of new techniques and treatments for the management of ophthalmic disease. Our research efforts are focused in the following three major areas:
- The study of new therapeutics addressing key causes of vision loss including age-related macular degeneration, diabetic retinopathy, ocular inflammation (uveitis), vitreomacular traction, retinopathy of prematurity, keratoconus, and the ocular complications of AIDS
- The development and evaluation of new surgical approaches and devices for age-related macular degeneration including implantable drug reservoirs, stem cell implantation and the implantable miniature telescope
- The testing of new ocular imaging technologies including wide-field Optos angiography and optical coherence tomographic angiography (OCT-A). Grant-funded by the Juvenile Diabetics Research Foundation, we employed the first OCT in Illinois
Our studies
Age-related macular degeneration (AMD)
- Safety and Efficacy of Abicipar Pegol in Patients with Neovascular Age-related Macular Degeneration: This is a multicenter, double-masked, randomized, 100-week, parallel-group, active controlled study to evaluate the safety and efficacy of abicipar in treatment-naïve patients with neovascular AMD. (currently enrolling)
- A Multicenter, Prospective Epidemiologic Study of the Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration, Proxima A: The primary objective of this observational study is to generate a better understanding of the variability and dynamic range over time of visual function tests in patients with geographic atrophy secondary to age-related macular degeneration (AMD). Secondary objectives include of this observational study include evaluation of the mean change in the GA area from baseline over time, examine rates of selected medical and ocular events in a patient population with GA secondary to AMD, and to evaluate changes over time in selected patient-reported outcomes in patients with GA secondary to AMD. (currently enrolling)
- Implantable Miniature Telescope (IMT) Imaging Study (currently enrolling)
- Post-Approval Study of Visioncare's Implantable Miniature Telescope (IMT) in Patients with Bilateral Severe to Profound Central Vision Impairment Associated with End-Stage Age-Related Macular Degeneration (currently enrolling)
- Evaluation of OCT-Angiography in Retinal Patients (currently enrolling)
Dry AMD
- A Phase II, Multicenter, Randomized, Single-Masked, Sham Injection-Controlled Exposure-Response Study of Lampalizumab Intravitreal Injections Administered Every Two Weeks or Every Four Weeks to Patients With Geographic Atrophy
Wet AMD
- Phase 3 study of the Efficacy and safety of Squalamine Lactate Ophthalmic Solution 0.2% administered twice daily in subjects with neovascular age-related macular degeneration
Diabetic Retinopathy
- Prompt Panretinal Photocoagulation versus Intravitreal Ranibizumab with Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy: The primary objective of the protocol is to determine if visual acuity outcomes at 2 years in eyes with PDR that receive anti-VEGF therapy with deferred PRP are non-inferior to those in eyes that receive standard prompt PRP therapy.
- Peripheral Diabetic Retinopathy Lesions on Ultrawide-field Fundus Images and Risk of Diabetic Retinopathy Worsening Over Time (Protocol AA)
- Intravitreous Anti-VEGF Treatment for Prevention of Vision Threatening Diabetic Retinopathy in Eyes at High Risk
Uveitis
- Multi-Center Uveitis Steroid Treatment (MUST) Trial: The objectives of this study are to compare the visual outcomes of patients with uveitis treated with the sustained release intraocular fluocinolone acetonide implant therapy compared to those treated with systemic therapy using oral corticosteroids supplemented by corticosteroid-sparing immunosuppressive drugs when indicated. The efficacy of the alternative treatment strategies for controlling uveitis and ameliorating/preventing structural complications of uveitis will be observed over time, the rates of local ocular corticosteroid-induced complications and the rates of systemic complications between the treatment groups, as well as the self-reported quality of life of the treatment groups will be examined.
- Macular Edema Treatment Trials Associated with MUST (META-MUST): To evaluate the relative efficacy of three commonly utilized regional corticosteroids for the regional treatment of uveitic macular edema: periocular triamcinolone acetonide; intravitreal triamcinolone acetonide; intravitreal dexamethasone implant. The primary efficacy measure will be percent change in central subfield thickness as measured by OCT at 8 weeks. Participants will continue in the study for 24 weeks in order to evaluate relative effects of the 3 treatment strategies on the duration of treatment effects, requirement for additional injections, and adverse effects. (currently enrolling)
- A Multicenter Open-Label Study of the Long-term Safety and Efficacy of the Human Anti-TNF Monoclonal Antibody Adalimumab in Subjects with Non-infectious Intermediate-, Posterior-, or Pan-uveitis: The primary objective of this study is to evaluate the long-term safety of adalimumab
40 mg given every other week (eow) subcutaneously (SC) in subjects with non-infectious intermediate-, posterior-, or pan-uveitis who participated in Study M10-877 or Study M10-880. Long-term efficacy will also be assessed.
Vitreomacular adhesion
- Ocriplasmin Research to Better Inform Treatment (ORBIT): To observe the clinical outcomes and safety in patients receiving JETREA® for the treatment of symptomatic vitreomacular adhesion (VMA) as per standard of care in US retina clinics.
Central serous chorio-retinopathy
- Rifampin for Central Serous Chorio-Retinopathy: This study will investigate the efficacy of oral rifampin for the treatment of
chronic central serous chorioretinopathy.The primary objective is to determine whether daily treatment with oral rifampin (600 mg or 300 mg) causes resolution of chronic retinal pigment epithelial (RPE) leakage and neurosensory retinal detachments (SRD) in patients with chronic CSC at months 3 and 6 of treatment. (currently enrolling)
Glaucoma
- A Randomized Study Comparing the Safety and Efficacy of the InnFocus MicroShunt Glaucoma Drainage System to Standard Trabeculectomy In Subjects with Primary Open Angle Glaucoma: The study objective is to assess the safety and effectiveness of the InnFocus MicroShunt when used to lower intraocular pressure (IOP) in subjects with primary open angle glaucoma where the IOP is not controlled when using maximum tolerated glaucoma medications.(currently enrolling)
Resident/student studies
- Selective Laser Trabeculoplasty in Patients with Elevated Intraocular Pressure after Descemet's Stripping Endothelial Keratoplasty or Penetrating Keratoplasty
- Descemet's Stripping Endothelial Keratoplasty in Glaucoma Patients Following Tube Shunt and/or Trabeculectomy
- Demographic Analysis of Patients with Retinopathy of Prematurity Who Required Treatment
- Zonular Instability in Autosomal Dominant Vitreoretinochoroidopathy
Contact us
Luma Halig, OD, MS
Luma_Halig@rush.edu