Radioactive materials and radiation-producing devices play an important role in medical research and teaching, as well as in the diagnosis and treatment of disease at RUSH University. The use of these materials and devices is authorized and governed by federal, state and local regulations, in addition to policies and procedures established by the university and its associated medical facilities.
Because radiation can be potentially hazardous to human health, RUSH has established its Radiation Safety Program, which supports the safe use of these tools by establishing structures and procedures to ensure that: any hazards associated with the use of radioactive materials or radiation-producing devices are minimized; and that all uses of these materials and devices are in compliance with applicable regulatory requirements.
-
Radiation Safety Program Oversight and Responsibilities
RUSH University Medical Center’s Radiation Safety Program operates under the direction of Radiation Safety Officer (RSO) William White and Radiation Safety Officer Heather Merchantz. Mark Supanich, PhD, chairs the program’s broad-based Radiation Safety Committee (RSC), as required by the state of Illinois. All routine clinical and research laboratory aspects of ionizing radiation use are managed by the RSO under the RSC’s oversight. Procedures governing the use of radioactive materials and analytical X-ray devices at RUSH University, as well as manuals, handbooks and applications for principal investigators (PIs) requesting the use of radioactive material in research labs can be found on Inside RUSH.
In addition, lead-apron garments for X-ray use must be inventoried and inspected on an annual basis. Questions and inquiries for lead apron garments should be directed to LeadApron_Check@rush.edu.
The RSC also is responsible for overseeing all uses of radioactive material. Therefore, all clinical and non-clinical uses of ionizing radiation for research purposes must be reviewed and approved by the RSC. All research studies using ionizing radiation for treatment, guidance or localization, screening or treatment-response assessment must be submitted to the RSC for radiation safety review. If the use of ionizing radiation is determined to be consistent with the prevailing Standard of Care (SOC), no further review is necessary. However, If the use of ionizing radiation differs from the standard of care or presents unknown or increased radiation risks, a designee of the RSC will review the protocol and informed-consent document and provide model radiation-risk consent language if required. Some protocols, such as new uses of radioactive materials, may require full RSC review.
-
Research Investigator Training
Investigators and research teams at RUSH primarily use ionizing radiation for X-rays, CT scans, radiation oncology and external radiation beams as they relate to clinical trials. Every research study is led by a project manager who determines the team’s department-specific training. The RUSH Learning Hub also provides training modules that may be required initially and/or annually as determined by the RSO. Research teams may ask the RSC to arrange for specific types of research training, or the RSC may reach out to researchers with training requests. In any case, two-way communication remains a constant, and investigators and teams are always welcome to reach out to the RSC at radiation_safety@rush.edu.
-
RSC Procedure for Review of Research Studies Using Ionizing Radiation on Human Subjects
The RSC delegates responsibility to a subset of RSC members to review clinical-research use of ionizing radiation. The review process is designed to ensure that the radiation risks associated with participating in various types of research are presented in language that is clear and easy for participants to understand. Any use of radiation in a research study requires researchers to fill out a Radiation Safety Review smart form by logging into the RUSH Research Portal. This is a web-based form with branching logic that walks investigators and their teams through the process of submitting required information to be reviewed by the RSC designee.
The various standard types of RSC-recommended radiation-risk language to include in informed consents for studies using ionizing radiation (differing in dose or physiological effect from the standard of care for individuals not participating in the research) are given below. A guidance document on completing the RSC Smart Form in the portal follows the model consent language.
- Therapeutic Studies Consent (i.e., radiation therapy, radiation embolization, surgical implantation of intracardiac devices, etc., where the radiation is inherent to the intervention)
If you participate in this study, you will be exposed to ionizing radiation (like X-rays) to treat your disease that differs in dose, timing and/or radiation type from the radiation that would be used in standard care for your condition. The radiation exposure is a necessary part of this experimental therapy. This experimental radiation therapy may not be better and could be worse at treating or controlling your disease than the standard-of-care approach. Ionizing radiation can contribute to a person’s natural risk of developing cancer (or a second cancer), and can cause additional side effects. Your study doctor will discuss those side effects with you. These risks should be considered together with other risks of study participation.
- Studies with concomitant radiation therapy and an agent that may amplify cell death
If you participate in this study, you will be exposed to ionizing radiation for therapeutic purposes, along with one or more companion agents that are expected to strengthen the radiation’s effects. The combination also is expected to strengthen the therapeutic effects of the radiation, but also may increase the severity or likelihood of radiation side effects. Your study doctor will discuss those possible side effects with you. These risks should be considered together with other risks of study participation.
- Studies using Image-Guided Interventions (i.e., fluoroscopy for localization, guidance, verification of implant placement or treatment)
If you participate in this study, you will undergo an image-guided procedure that uses ionizing radiation (X-ray fluoroscopy). There is a rare risk of skin injury from the use of fluoroscopy. The risk depends on the complexity of the procedure and factors such as the part of the body being imaged and other health issues that you may have. Following the X-ray fluoroscopy procedure, the skin area exposed to X-rays could redden and initially feel like a sunburn. If a skin reaction occurs, it could appear after a few hours or up to a few weeks after the procedure. It usually goes away on its own, but it is possible that the reaction could be long-lasting or permanent. If your doctor determines that you have a significant risk of developing a skin injury because of the complexity and length of your procedure, additional information will be given to you. If you have a skin reaction, you should tell the study doctor immediately, and you may be asked to return for a study visit.
- Consent for all other studies with radiation exposure less than 100mSv or studies using RECIST 1.1 (or similar criteria)
If you participate in this study, you will receive imaging exams that use ionizing radiation (like X-rays) in addition to, or more frequently than, the imaging you might receive if you do not participate. Exposure to ionizing radiation from these additional imaging exams may add slightly to a person’s natural risk of developing cancer or a second cancer.
- Consent for all other studies (i.e, non-RECIST 1.1, with exposure more than 100mSv over the course of study)
If you participate in this study, you will receive imaging exams that use ionizing radiation (like X-rays) in addition to, or more frequently than, the imaging you might receive if you do not participate. These additional imaging exams typically include CT scans or PET scans. Exposure to ionizing radiation from additional imaging exams may add slightly to a person’s natural risk of developing cancer or a second cancer. In populations exposed to similar amounts of radiation, the observed average excess risk is at least 0.6 percent, or 1 chance in 167, over a normal lifetime. Your own risk of developing cancer from exposure to the additional radiation required by this study may be lesser or greater, depending on factors including age, genetics, tobacco use and environmental factors.
- Therapeutic Studies Consent (i.e., radiation therapy, radiation embolization, surgical implantation of intracardiac devices, etc., where the radiation is inherent to the intervention)
-
Radiation Safety Review Process Guidance
To complete the RSC smart form on the RUSH Research Portal, you'll follow the following steps.
Project-Specific Information in the Master Project
Answering Yes to question 6.0 – "Does this study use ionizing radiation (such as X-ray, CT, fluoroscopy, radioactive materials, or radiation therapy)?" – will open the Radiation Safety module for completion and activate the Create Radiation Safety button in your left task bar.
Image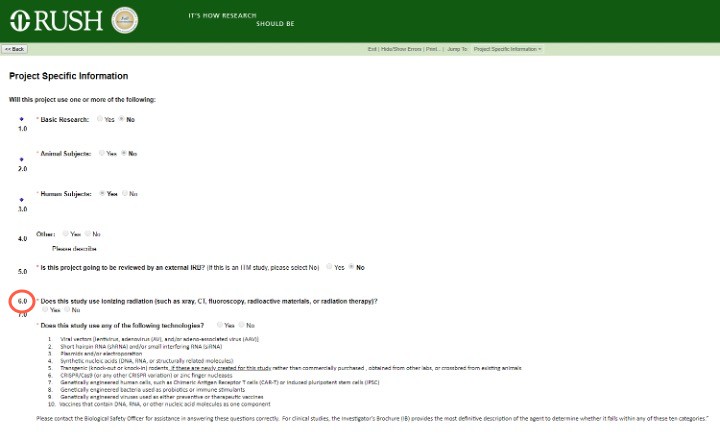
Radiation Safety Module
Image
To create a new Radiation Safety application, click on the Create Radiation Safety button on the left task bar.
Image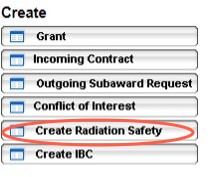
Dose of Radiation
Image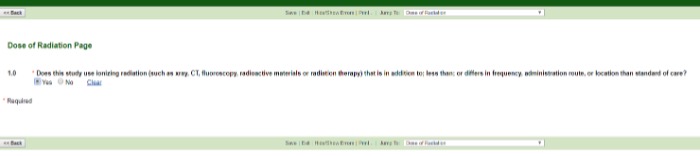
If the dose differs (i.e., lower, higher, different location, more frequently, different administration route) from the SOC, check Yes. If the dose does not differ, check No and complete the final application questions for review.
A good rule of thumb for determining CT imaging SOC: 1X every 3 months is a typical SOC regime for CT imaging.
Typically, a single chest X-ray is SOC. More than one X-ray would be an increase in radiation dose.
Type of Ionizing Radiation (that deviates from the standard of care)
Image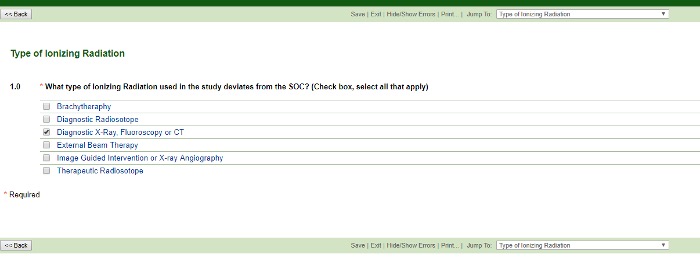
Think about the campus location where the procedure will occur to determine the type of radiation. For example, if the procedure will occur on the 4th or 5th floor of the Tower, it will most likely be an image-guided intervention.
You may select more than one type. Based on your choices above, the application will branch out correspondingly. Choosing Brachytherapy, Diagnostic Radioisotope, External Beam Therapy or Therapeutic Radioisotope choices branch to reveal:
Image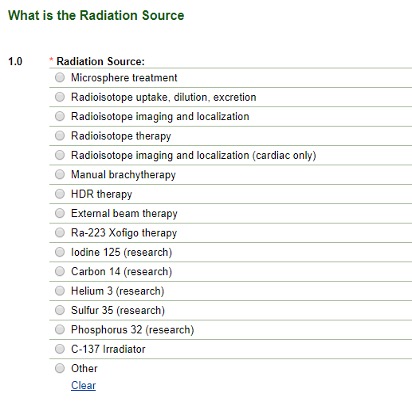
Only one radio button may be selected on this screen.
Approved Authorized User
Image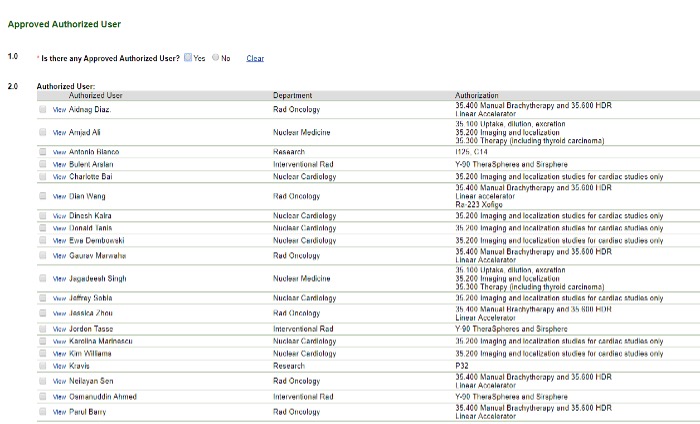
If there is not an approved authorized user listed or if you don't see the user you need, email radiation_safety@rush.edu.
Use of Radiation (that deviates from the standard of care)
Image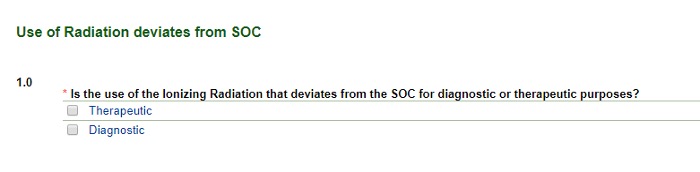
- Selecting Therapeutic takes you to text boxes where you will explain the radiation therapy approach for the research and the SOC. Copy and paste a few sentences from the protocol. (Including the page numbers from the protocol will be useful here.)
- Selecting Diagnostic reveals the following screen. Add all of the radiologic procedures used in the study that are not SOC first. If the protocol is open-ended, use a two-year timeframe. You will be prompted to enter the SOC radiologic procedures next.
Image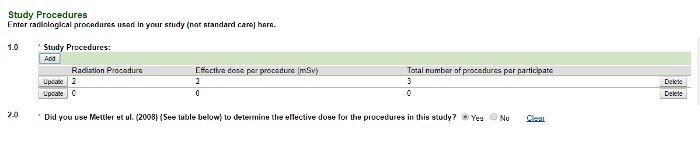 Image
Image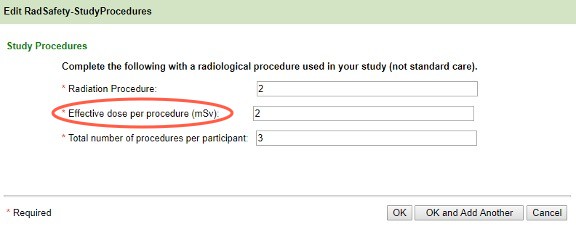 Image
Image Image
Image Image
Image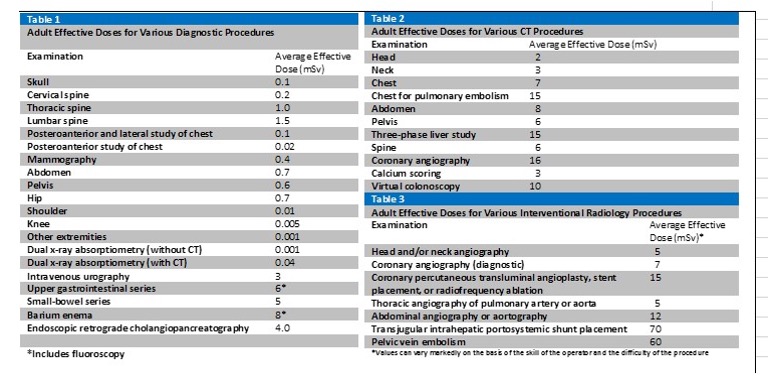 Image
Image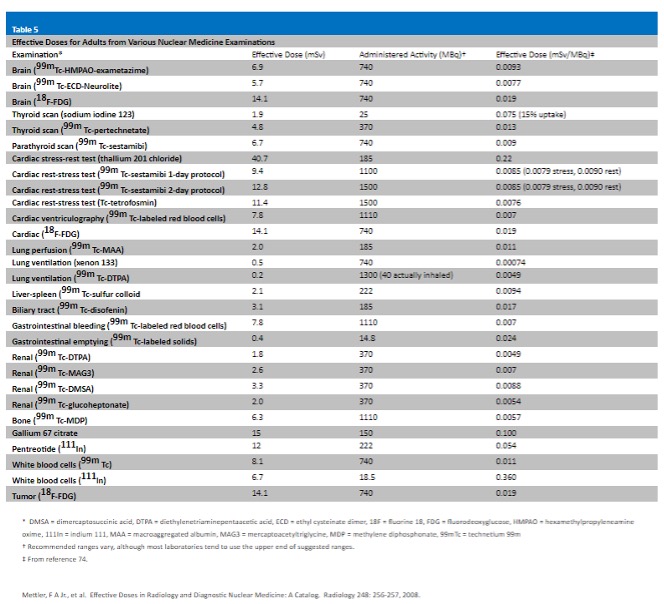
Radiation Dose Calculations are determined from the radiation dose and subject information entered. You will be asked if you accept the calculated average excess risk.
- If you select No, you will be required to enter an alternate risk (a numeric value) and an alternate calculation.
- If you select Yes, you will see and complete one of the following fields.
Consent for Studies
These questions provide the template ICF language to use. Please adapt it to your study, and enter the final version into the text box and your ICF for use.
Image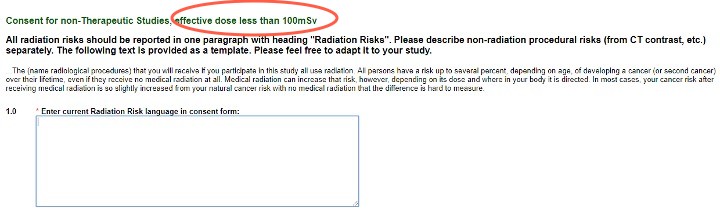
Image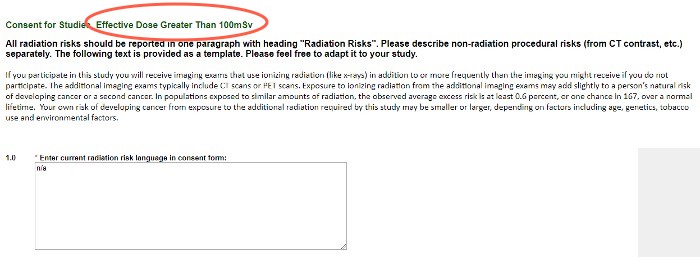
Investigator Comments
Image
Please enter a few sentences about what the radiation is. Denoting the page number in the protocol where radiation is discussed would be useful. Also, include the page number of the schedule of events here.
Type of Ionizing Radiation
Image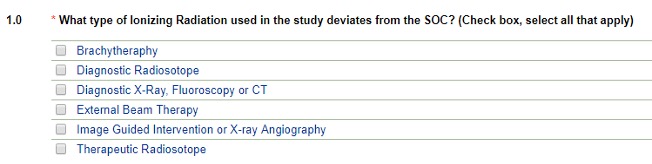
- Selecting Diagnostic X-Ray, Fluoroscopy or CT will branch to the diagnostic pathway (see above)
- Selecting Image-Guided Intervention or X-ray Angiography will display the following fields:
Image Image
Image
This question provides the template ICF language to use. Please adapt it to your study, and enter the final version into the text box and your ICF for use.
It is possible to select more than one type of ionizing radiation used in your study that deviates from the SOC. This will send you down individual pathways as above.
Some pathways will inform you that a Radiation Safety Committee member needs to review the protocol to suggest the appropriate Informed Consent Form (ICF) language. Different language is presented for different-use scenarios as you navigate the module. These ICF language suggestions will be communicated directly to the individual submitting via the RUSH Research Portal. Please adapt the recommended language specifically to your study.
Heather Merchantz
Radiation Safety Officer
radiation_safety@rush.edu
